Abstract
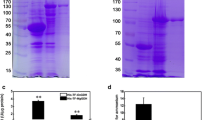
Carbonic anhydrate is a zinc-containing metalloenzyme and involved in plant abiotic stress tolerance. In this study, we found that heat stress could induce rice mature carbonic anhydrate gene over-expression in rice plants. An Escherichia coli heterologous expression system was performed to identify the function of rice mature carbonic anhydrate in vitro. By sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), mature OsCA fusion protein was identified and proved to be soluble. The results of spot, survival rate, and growth curve assay demonstrated that the expression of the mature OsCA could enhance the thermo-tolerance of the induced mature OsCA recombinants in comparison with controls under heat stress. Meanwhile, compared with controls, the levels of reactive oxygen species in induced mature OsCA recombinants were apparently low under heat stress, and correspondingly, activities of the critical antioxidant enzymes including superoxide dismutase, catalase, and peroxidase in the induced mature OsCA recombinants were significantly increased. Additionally, relative to controls, the activity of the lactate dehydrogenase decreased in the induced mature OsCA recombinants under heat stress. Based on these results, we suggest that mature OsCA protein could confer the E. coli recombinants’ tolerance to heat stress by a synergistic fashion of increasing the antioxidant enzymes’ activities to reduce the oxidative damage and maintaining the lactate dehydrogenase (LDH) activity of E. coli.




Similar content being viewed by others
References
Craufurd, P. Q., Jagadish, S. V. K., Wheeler, T. R., Bennett, J., & Lafitte, R. (2007). Heat tolerance at flowering. Abstract: An international workshop on “Cool rice for a warmer world”, Wuhan, China.
Liu, Y., Zheng, Y. Z., Zhang, Y. Q., Wang, W. M., & Li, R. H. (2010). Soybean PM2 protein (LEA3) confers the tolerance of Escherichia coli and stabilization of enzyme activity under diverse stresses. Current Microbiology, 60, 373–378.
Lobell, D. B., & Asner, G. P. (2003). Climate and management contributions to recent trends in U.S. agricultural yields. Science, 299, 1032.
Zhang, G. L., Chen, L. Y., Lei, D. Y., & Zhang, S. T. (2005). The research progress of heat resistance in rice. Hybrid Rice, 20, 1–5.
Supuran, C. T. (2008). Carbonic anhydrases—an overview. Current Pharmaceutical Design, 14, 603–614.
Tiwari, A., Kumar, P., Singh, S., & Ansari, S. A. (2005). Carbonic anhydrase in relation to higher plants. Photosynthetica, 43, 1–11.
Karlsson, J., Clarke, A. K., Chen, Z. Y., Hugghins, S. Y., Park, Y. I., Husic, H. D., Moroney, J. V., & Samuelsson, G. (1998). A novel α-type carbonic anhydrase associated with the thylakoid membrane in Chlamydomonas reinhardtii is required for growth at ambient CO2. EMBO Journal, 17, 1208–1216.
Moskvin, O. V., Ivanov, B. N., Ignatova, L. K., & Kollmeier, M. A. (2000). Light-induced stimulation of carbonic anhydrase activity in pea thylakoids. FEBS Letters, 470, 375–377.
Kisiel, W., & Graf, G. (1972). Purification and characterization of carbonic anhydrase from Pisum sativum. Phytochemistry, 11, 113–117.
Chang, C. W. (1975). Activation energy for thermal inactivation and Km of carbonic anhydrase from the cotton plant. Plant Science Letters, 4, 109–113.
Lazova, G. N., Kicheva, M. I., & Popova, L. P. (2000). The effect of abscisic acid and methyl jasmonate on carbonic anhydrase activity in pea. Photosynthetica, 36, 631–634.
Yu, S., Zhang, X. X., Guan, Q. J., Takano, T., & Liu, S. K. (2007). Expression of a carbonic anhydrase gene is induced by environmental stresses in rice (Oryza sativa L.). Biotechnology Letters, 29, 89–94.
Kaul, T., Reddy, P. S., Mahanty, S., Thirulogachandar, V., Reddy, R. A., Kumar, B., Sopory, S., & Reddy, M. K. (2011). Biochemical and molecular characterization of stress-induced β-carbonic anhydrase from a C4 plant, Pennisetum glaucum. Journal of Plant Physiology, 168, 601–610.
Bageshwar, U. K., Premkumar, L., Gokhman, I., Savchenko, T., Sussman, J., & Zamir, A. (2004). Natural protein engineering: a uniquely salt-tolerant, but not halophilic, α-type carbonic anhydrase from algae proliferating in low-to hyper-saline environments. Protein Engineering Design and Selection, 17, 191–200.
Liu, Y., & Zheng, Y. Z. (2005). PM2, a group 3 LEA protein from soybean, and its 22-mer repeating region confer salt tolerance in Escherichia coli. Biochemical and Biophysical Research Communications, 331, 325–332.
Pan, J., Wang, J., Zhou, Z. F., Yan, Y. L., Zhang, W., Lu, W., Ping, S. Z., Dai, Q. L., Yuan, M. L., Feng, B., Hou, X. G., Zhang, Y., Ma, R. Q., Liu, T. T., Feng, L., Wang, L., Chen, M., & Lin, M. (2009). IrrE, a global regulator of extreme radiation resistance in Deinococcus radiodurans, enhances salt tolerance in Escherichia coli and Brassica napus. PLoS One, 4, e4422.
Tanaka, S., Ikeda, K., Ono, M., & Miyasaka, H. (2002). Isolation of several anti-stress genes from a mangrove plant Avicennia marina. World Journal of Microbiology and Biotechnology, 18, 801–804.
Garay-Arroyo, A., Colmenero-Flores, J. M., Garciarrubio, A., & Covarrubias, A. A. (2000). High hydrophilic proteins in prokaryotes and eukaryotes are common during conditions of water deficit. Journal of Biological Chemistry, 275, 5668–5674.
Bai, Z., Harvey, L. M., & McNeil, B. (2003). Elevated temperature effects on the oxidant/antioxidant balance in submerged batch cultures of the filamentous fungus Aspergillus niger B1-D. Biotechnology and Bioengineering, 83, 772–779.
Abrashev, R. I., Pashova, S. B., Stefanova, L. N., Vassilev, S. V., Dolashka-Angelova, P. A., & Angelova, M. B. (2008). Heat-shock-induced oxidative stress and antioxidant response in Aspergillus niger 26. Canadian Journal of Microbiology, 54, 977–983.
Kim, I. S., Moon, H. Y., Yun, H. S., & Jin, I. (2006). Heat shock causes oxidative stress and induces a variety of cell rescue proteins in Saccharomyces cerevisiae KNU5377. Journal of Microbiology, 44, 492–501.
Belozerskaya, T. A., & Gessler, N. N. (2007). Reactive oxygen species and the strategy of antioxidant defense in fungi: a review. Applied Biochemistry and Microbiology, 43, 506–515.
Begonia, G. B., & Salin, M. L. (1991). Elevation of superoxide dismutase in Halobacterium halobium by heat shock. Journal of Bacteriology, 173, 5582–5584.
Romandini, P., Bonotto, C., Bertoloni, G., Beltramini, M., & Salvato, B. (1994). Superoxide dismutase, catalase and cell dimorphism in Candida albicans cells exposed to methanol and different temperatures. Comparative Biochemistry and Physiology. Pharmacology, Toxicology and Endocrinology, 108, 53–57.
Acknowledgments
This research was partly supported by the National Transgenic Research and Development Program (2011ZX08001-004) and the 863 program (31071391) of China.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
The amino acid sequences of the β-type carbonic anhydrate (OsCA). It contains a chloroplast transit peptide of 63 amino acid residues and a mature protein of 210 amino acid residues (GIF 133 kb)
Rights and permissions
About this article
Cite this article
Tianpei, X., Mao, Z., Zhu, Y. et al. Expression of Rice Mature Carbonic Anhydrase Gene Increase E. coli Tolerance to Heat Stress. Appl Biochem Biotechnol 176, 625–635 (2015). https://doi.org/10.1007/s12010-015-1600-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1600-8