Abstract
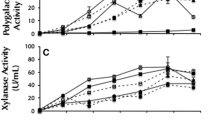
Hydrolytic enzymes were evaluated on the lipid accumulation via an oleaginous fungal species, Mortierella isabellina, cultivated on sugars released from soybean hulls. The weight loss of soybean hull, fungal growth, and lipid production were tested under different loads of hydrolytic enzymes. M. isabellina could not directly utilize cellulose and adding cellulase and β-glucosidase significantly increased the cell growth and oil accumulation of M. isabellina on soybean hulls. The highest weight loss of soybean hulls was 47.80 % and the lipid production reached 0.14 g from 1 g of soybean hull when 12 U cellulase, 27.2 U β-glucosidase, 2,278.56 U pectinase, and 15 U hemicellulase were added. Fatty acids (76.82 %) accumulated in M. isabellina were C16 and C18, which are suitable for biodiesel production. These results provide a new application for soybean hulls to be applied as the raw material for the production of biodiesel fuel, besides its traditional role as animal feed supplements.






Similar content being viewed by others
References
LAW, P. (2007). Energy independence and security act of 2007.
Zhang, J. G., & Hu, B. (2012). Solid-state fermentation of Mortierella isabellina for lipid production from soybean hull. Applied Biochemistry and Biotechnology, 166(4), 1034–1046.
Dey, P., Banerjee, J., & Maiti, M. K. (2011). Comparative lipid profiling of two endophytic fungal isolates—Colletotrichum sp. and Alternaria sp. having potential utilities as biodiesel feedstock. Bioresource Technology, 102(10), 5815–5823.
Subramaniam, R., et al. (2010). Microbial lipids from renewable resources: production and characterization. Journal of Industrial Microbiology & Biotechnology, 37(12), 1271–1287.
Heredia-Arroyo, T., Wei, W., & Hu, B. (2010). Oil accumulation via heterotrophic/mixotrophic Chlorella protothecoides. Applied Biochemistry and Biotechnology, 162(7), 1978–1995.
Xia, C., et al. (2011). A new cultivation method for microbial oil production: cell pelletization and lipid accumulation by Mucor circinelloides. Biotechnology for biofuels, 4(1), 15.
Sharma, R. K., & Arora, D. S. (2010). Production of lignocellulolytic enzymes and enhancement of in vitro digestibility during solid state fermentation of wheat straw by Phlebia floridensis. Bioresource Technology, 101(23), 9248–9253.
Krishna, C. (2005). Solid-state fermentation systems—an overview. Critical Reviews in Biotechnology, 25(1–2), 1–30.
Meng, X., et al. (2009). Biodiesel production from oleaginous microorganisms. Renewable Energy, 34(1), 1–5.
Zhang, J. A., et al. (2010). Biodetoaxification of toxins generated from lignocellulose pretreatment using a newly isolated fungus, Amorphotheca resinae ZN1, and the consequent ethanol fermentation. Biotechnology For Biofuels, 3, 26.
Masuda, T., & Goldsmith, P. D. (2009). World soybean production: area harvested, yield, and long-term projections. International Food and Agribusiness Management Review, 12(4), 143–161.
Mielenz, J. R., Bardsley, J. S., & Wyman, C. E. (2009). Fermentation of soybean hulls to ethanol while preserving protein value. Bioresource Technology, 100(14), 3532–3539.
Yoo, J., et al. (2011). Thermo-mechanical extrusion pretreatment for conversion of soybean hulls to fermentable sugars. Bioresource Technology, 102(16), 7583–7590.
Tuomela, M., Hatakka, M. V. A., & Itävaara, M. (2000). Biodegradation of lignin in a compost environment: a review. Bioresource Technology, 72, 169–183.
Kumar, P., et al. (2009). Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Industrial and Engineering Chemistry Research, 48(8), 3713–3729.
Palmqvist, E., & Hahn-Hägerdal, B. (2000). Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Bioresource Technology, 74, 25–33.
Kumar, D., & Murthy, G. S. (2011). Impact of pretreatment and downstream processing technologies on economics and energy in cellulosic ethanol production. Biotechnology for Biofuels, 4, 27.
Suzuki, O., (1990). Recent trends of oleochemicals by biotechnology. World Conference on Oleochemicals, pp. 221–230.
Meeuwse, P., Tramper, J., & Rinzema, A. (2011). Modeling lipid accumulation in oleaginous fungi in chemostat cultures: I. Development and validation of a chemostat model for Umbelopsis isabellina. Bioprocess and Biosystems Engineering, 34, 939–949.
Meeuwse, P., Tramper, J., & Rinzema, A. (2011). Modeling lipid accumulation in oleaginous fungi in chemostat cultures. II: validation of the chemostat model using yeast culture data from literature. Bioprocess and Biosystems Engineering, 34, 951–961.
Roopesh, K., et al. (2006). Comparison of phytase production on wheat bran and oilcakes in solid-state fermentation by Mucor racemosus. Bioresource Technology, 97(3), 506–511.
Ho, N. W. Y., Chen, Z. D., & Brainard, A. P. (1998). Genetically engineered Sacccharomyces yeast capable of effective cofermentation of glucose and xylose. Applied and Environmental Microbiology, 64(5), 1852–1859.
Chatzifragkou, A., et al. (2010). Commercial sugars as substrates for lipid accumulation in Cunninghamella echinulata and Mortierella isabellina fungi. European Journal of Lipid Science and Technology, 112(9), 1048–1057.
Papanikolaou, S., et al. (2007). Lipid production by oleaginous Mucorales cultivated on renewable carbon sources. European Journal of Lipid Science and Technology, 109(11), 1060–1070.
Sharma, K. K., Schuhmann, H., & Schenk, P. M. (2012). High lipid induction in microalgae for biodiesel production. Energies, 5(5), 1532–1553.
Heredia-Arroyo, T., et al. (2011). Mixotrophic cultivation of Chlorella vulgaris and its potential application for the oil accumulation from non-sugar materials. Biomass and Bioenergy, 35(5), 2245–2253.
Ratledge, C. (2008). Microbial lipids. In H.-J. Rehm & G. Reed (Eds.), Biotechnology: products of secondary metabolism (pp. 133–197). Weinheim: Wiley.
Excoffier, G., Toussaint, B., & Vignon, M. R. (1991). Saccharification of steam-exploded poplar wood. Biotechnology and Bioengineering, 38(11), 1308–1317.
Xin, Z., Yinbo, Q., & Peiji, G. (1993). Acceleration of ethanol-production from paper-mill waste fiber by supplementation with beta-glucosidase. Enzyme and Microbial Technology, 15(1), 62–65.
Ghose, T. K., & Bisaria, V. S. (1979). Studies on the mechanism of enzymatic-hydrolysis of cellulosic substances. Biotechnology and Bioengineering, 21(1), 131–146.
Beldman, G., et al. (1984). Application of cellulase and pectinase from fungal origin for the liquefaction and saccharification of biomass. Enzyme and Microbial Technology, 6(11), 503–507.
Zhang, M. J., et al. (2010). Enhanced enzymatic hydrolysis of lignocellulose by optimizing enzyme complexes. Applied Biochemistry and Biotechnology, 160(5), 1407–1414.
Ye Sun, J. C. (2002). Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresource Technology, 83, 1–11.
Liu, L., et al. (2009). Enhanced enzymatic hydrolysis and structural features of corn stover by FeCl(3) pretreatment. Bioresource Technology, 100(23), 5853–5858.
Knothe, G. (2009). Improving biodiesel fuel properties by modifying fatty ester composition. Energy & Environmental Science, 2(7), 759–766.
Pinzi, S., Garcia, I. L., Lopez-Gimenez, F. J., de Castro, M. D. L., Dorado, G., & Dorado, M. P. (2009). The ideal vegetable oil-based biodiesel composition: a review of social, economical and technical implications. Energy & Fuels, 23, 2325–2341.
Acknowledgments
Dr. Jianguo Zhang's research was supported by Dr. Bo Hu's faculty seed money program at University of Minnesota. Part of the work was also supported by Grant-in-Aid program at the University of Minnesota.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, J., Hu, B. Effects of External Enzymes on the Fermentation of Soybean Hulls to Generate Lipids by Mortierella isabellina . Appl Biochem Biotechnol 168, 1896–1906 (2012). https://doi.org/10.1007/s12010-012-9905-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-9905-3