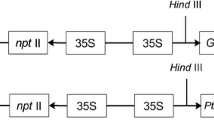
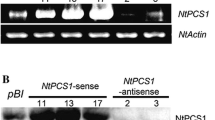
Abstract
Phytochelatin synthase (PCS) is a key enzyme involved in the synthesis of phytochelatins, which are thought to play important roles in heavy metal detoxification. The sacred lotus (Nelumbo nucifera), one of the most popular ornamental species, has been shown to be a potential phytoremediator of heavy metal polluted water. However, the phytochelatin synthase gene in N. nucifera has not been identified yet. Here, we report the isolation and function characterization of a N. nucifera homologue of phytochelatin synthase. The sequence obtained shares a high degree of similarity with PCSs from other plant species and was named as Nelumbo nucifera phytochelatin synthase1 (NnPCS1). By using quantitative RT-PCR, we found that the expression of NnPCS1 in leaves of N. nucifera was dramatically increased in response to Cadmium (Cd) treatment. We further showed that, when exposed to Cd stress, Arabidopsis transgenic plants heterologous expressing NnPCS1 accumulated more Cd when compared with wild type. These results suggest that NnPCS1 involved in the response of N. nucifera to Cd stress and may represent a useful target gene for the phytoremediation of Cd-polluted water.







Similar content being viewed by others
References
Brulle, F., Cocquerelle, C., Wamalah, A. N., Morgan, A. J., Kille, P., Leprêtre, A., & Vandenbulcke, F. (2008). cDNA cloning and expression analysis of Eisenia fetida (Annelida: Oligochaeta) phytochelatin synthase under cadmium exposure. Ecotoxicology and Environmental Safety, 71, 47–55.
Liu, J., Dong, Y., Xu, H., Wang, D., & Xu, J. (2007). Accumulation of Cd, Pb and Zn by 19 wetland plant species in constructed wetland. Journal of Hazardous Materials, 147, 947–953.
Arao, T., Ae, N., Sugiyama, M., & Takahashi, M. (2003). Genotypic differences in cadmium uptake and distribution in soybeans. Plant and Soil, 251, 247–253.
Salt, D. E., Prince, R. C., Pickering, I. J., & Raskin, I. (1995). Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiology, 109, 1427–1433.
Grill, E., Winnacker, E. L., & Zenk, M. H. (1985). Phytochelatins: the principal heavy-metal complexing peptides of higher plants. Science, 230, 674–676.
Cobbett, C. S. (1999). A family of phytochelatin synthase genes from plant, fungal and animal species. Trends in Plant Science, 4, 335–337.
Cobbett, C., & Goldsbrough, P. (2002). Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annual Review of Plant Biology, 53, 159–182.
Kondo, N., Imai, K., Isobe, M., Goto, T., Murasugi, A., Wada-Nakagawa, C., & Hayashi, Y. (1984). Cadystin a and b, major unit peptides comprising cadmium binding peptides induced in a fission yeast—separation, revision of structures and synthesis. Tetrahedron Letters, 25, 3869–3872.
Hirata, K., Tsuji, N., & Miyamoto, K. (2005). Biosynthetic regulation of phytochelatins, heavy metal-binding peptides. Journal of Bioscience and Bioengineering, 100, 593–599.
Grill, E., Löffler, S., Winnacker, E. L., & Zenk, M. H. (1989). Phytochelatins, the heavy-metal-binding peptides of plants, are synthesized from glutathione by a specific γ—glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase). Proceedings of the National Academy of Sciences of the United States of America, 86, 6838–6842.
Clemens, S., Kim, E. J., Neumann, D., & Schroeder, J. I. (1999). Tolerance to toxic metals by a gene family of phytochelatin synthases from plants and yeast. The EMBO Journal, 18, 3325–3333.
Ha, S. B., Smith, A. P., Howden, R., Dietrich, W. M., Bugg, S., O'Connell, M. J., Goldsbrough, P. B., & Cobbett, C. S. (1999). Phytochelatin synthase genes from Arabidopsis and the yeast Schizosaccharomyces pombe. The Plant Cell Online, 11, 1153–1163.
Vatamaniuk, O. K., Mari, S., Lu, Y. P., & Rea, P. A. (1999). AtPCS1, a phytochelatin synthase from Arabidopsis: isolation and in vitro reconstitution. Proceedings of the National Academy of Sciences of the United States of America, 96, 7110–7115.
Oven, M., Page, J. E., Zenk, M. H., & Kutchan, T. M. (2002). Molecular characterization of the homo-phytochelatin synthase of soybean Glycine max: relation to phytochelatin synthase. Journal of Biological Chemistry, 277, 4747.
Dunbabin, J. S., & Bowmer, K. H. (1992). Potential use of constructed wetlands for treatment of industrial wastewaters containing metals. Science of the Total Environment, 111, 151–168.
Demrezen, D., & Aksoy, A. (2004). Accumulation of heavy metals in Typha angustifolia (L.) and Potamogeton pectinatus (L.) living in Sultan Marsh (Kayseri, Turkey). Chemosphere, 56, 685–696.
Weis, J. S., & Weis, P. (2004). Metal uptake, transport and release by wetland plants: implications for phytoremediation and restoration. Environment International, 30, 685–700.
Vajpayee, P., Sharma, S., Tripathi, R., Rai, U., & Yunus, M. (1999). Bioaccumulation of chromium and toxicity to photosynthetic pigments, nitrate reductase activity and protein content of Nelumbo nucifera gaertin. Chemosphere, 39, 2159–2169.
Miao, H., Jiang, B., Chen, S., Zhang, S., Chen, F., Fang, W., Teng, N., & Guan, Z. (2010). Isolation of a gibberellin 20-oxidase cDNA from and characterization of its expression in chrysanthemum. Plant Breeding, 128, 1–8.
Zhang, H., Xu, W., Guo, J., He, Z., & Ma, M. (2005). Coordinated responses of phytochelatins and metallothioneins to heavy metals in garlic seedlings. Plant Science, 169, 1059–1065.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods, 25, 402–408.
Gu, C., Chen, S., Liu, Z., Shan, H., Luo, H., Guan, Z., & Chen, F. (2011). Reference gene selection for quantitative real-time PCR in chrysanthemum subjected to biotic and abiotic stress. Molecular Biotechnology, 1, 1–6.
Ma, Y., Lin, S. Q., Gao, Y., Li, M., Luo, W. X., Zhang, J., & Xia, N. S. (2003). Expression of ORF2 partial gene of hepatitis E virus in tomatoes and immunoactivity of expression products. World Journal of Gastroenterology, 9, 2211–2215.
Clough, S. J., & Bent, A. F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal, 16, 735–743.
Jefferson, R. A., Kavanagh, T. A., & Bevan, M. W. (1987). GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. The EMBO Journal, 6, 3901–3907.
Lee, S., Moon, J. S., Ko, T. S., Petros, D., Goldsbrough, P. B., & Korban, S. S. (2003). Overexpression of Arabidopsis phytochelatin synthase paradoxically leads to hypersensitivity to cadmium stress. Plant Physiology, 131, 656–663.
Heiss, S., Wachter, A., Bogs, J., Cobbett, C., & Rausch, T. (2003). Phytochelatin synthase (PCS) protein is induced in Brassica juncea leaves after prolonged Cd exposure. Journal of Experimental Botany, 54, 1833.
Gasic, K., & Korban, S. S. (2007). Transgenic Indian mustard (Brassica juncea) plants expressing an Arabidopsis phytochelatin synthase (AtPCS1) exhibit enhanced As and Cd tolerance. Plant Molecular Biology, 64, 361–369.
Gasic, K., & Korban, S. S. (2007). A plant genetically modified that accumulates Pb is especially promising for phytoremediation. Planta, 225, 1277–1285.
Gisbert, C., Ros, R., De Haro, A., Walker, D. J., Pilar Bernal, M., Serrano, R., & Navarro-Aviñó, J. (2003). A plant genetically modified that accumulates Pb is especially promising for phytoremediation. Biochemical and Biophysical Research Communications, 303, 440–445.
Martinez, M., Bernal, P., Almela, C., Vélez, D., García-Agustín, P., Serrano, R., & Navarro-Avino, J. (2006). An engineered plant that accumulates higher levels of heavy metals than Thlaspi caerulescens, with yields of 100 times more biomass in mine soils. Chemosphere, 64, 478–485.
Guo, J., Dai, X., Xu, W., & Ma, M. (2008). Overexpressing GSH1 and AsPCS1 simultaneously increases the tolerance and accumulation of cadmium and arsenic in Arabidopsis thaliana. Chemosphere, 72, 1020–1026.
Maier, T., Yu, C., Küllertz, G., & Clemens, S. (2003). Localization and functional characterization of metal-binding sites in phytochelatin synthases. Planta, 218, 300–308.
Lee, S., & Korban, S. S. (2002). Transcriptional regulation of Arabidopsis thaliana phytochelatin synthase (AtPCS1) by cadmium during early stages of plant development. Planta, 215, 689–693.
Meuwly, P., & Rauser, W. E. (1992). Alteration of thiol pools in roots and shoots of maize seedlings exposed to cadmium: adaptation and developmental cost. Plant Physiology, 99, 8–15.
Zhu, Y. L., Pilon-Smits, E. A. H., Tarun, A. S., Weber, S. U., Jouanin, L., & Terry, N. (1999). Cadmium tolerance and accumulation in Indian mustard is enhanced by overexpressing gamma-glutamylcysteine synthetase. Plant Physiology, 121, 1169–1171.
Wawrzyński, A., Kopera, E., Wawrzyńska, A., Kamińska, J., Bal, W., & Sirko, A. (2006). Effects of simultaneous expression of heterologous genes involved in phytochelatin biosynthesis on thiol content and cadmium accumulation in tobacco plants. Journal of Experimental Botany, 57, 2173–2182.
Li, J., Guo, J., Xu, W., & Ma, M. (2006). Enhanced cadmium accumulation in transgenic tobacco expressing the phytochelatin synthase gene of Cynodon dactylon L. Journal of Integrative Plant Biology, 48, 928–937.
Pomponi, M., Censi, V., Di Girolamo, V., De Paolis, A., di Toppi, L. S., Aromolo, R., Costantino, P., & Cardarelli, M. (2006). Overexpression of Arabidopsis phytochelatin synthase in tobacco plants enhances Cd(2+) tolerance and accumulation but not translocation to the shoot. Planta, 223, 180–190.
Acknowledgments
This study is supported by the National Natural Science Foundation of China (grant no. 30872064, 31071820, 31071825), the Program for Hi-Tech Research, Jiangsu, China, (grant no. BE2008307, BE2009317, BE2010303) and the Fundamental Research Funds for the Central Universities (KYJ 200907).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Z., Gu, C., Chen, F. et al. Heterologous Expression of a Nelumbo nucifera Phytochelatin Synthase Gene Enhances Cadmium Tolerance in Arabidopsis thaliana . Appl Biochem Biotechnol 166, 722–734 (2012). https://doi.org/10.1007/s12010-011-9461-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-011-9461-2