Abstract
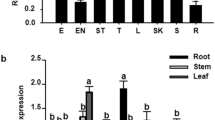
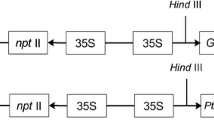
Phytochelatins (PCs) are metal binding peptides involved in heavy metal detoxification. To assess whether enhanced phytochelatin synthesis would increase heavy metal tolerance and accumulation in plants, we overexpressed the Arabidopsis phytochelatin synthase gene (AtPCS1) in the non-accumulator plant Nicotiana tabacum. Wild-type plants and plants harbouring the Agrobacterium rhizogenes rolB oncogene were transformed with a 35S AtPCS1 construct. Root cultures from rolB plants could be easily established and we demonstrated here that they represent a reliable system to study heavy metal tolerance. Cd2+ tolerance in cultured rolB roots was increased as a result of overexpression of AtPCS1, and further enhanced when reduced glutathione (GSH, the substrate of PCS1) was added to the culture medium. Accordingly, HPLC analysis showed that total PC production in PCS1-overexpressing rolB roots was higher than in rolB roots in the presence of GSH. Overexpression of AtPCS1 in whole seedlings led to a twofold increase in Cd2+ accumulation in the roots and shoots of both rolB and wild-type seedlings. Similarly, a significant increase in Cd2+ accumulation linked to a higher production of PCs in both roots and shoots was observed in adult plants. However, the percentage of Cd2+ translocated to the shoots of seedlings and adult overexpressing plants was unaffected. We conclude that the increase in Cd2+ tolerance and accumulation of PCS1 overexpressing plants is directly related to the availability of GSH, while overexpression of phytochelatin synthase does not enhance long distance root-to-shoot Cd2+ transport.







Similar content being viewed by others
Abbreviations
- DAG:
-
Days after germination
- GSH:
-
Glutathione
- PC:
-
Phytochelatin
References
Bellincampi D, Cardarelli M, Zaghi D, Serino G, Salvi G, Gatz C, Cervone F, Altamura MM, Costantino P, De Lorenzo G (1996) Oligogalacturonides prevent rhizogenesis in rolB-transformed tobacco explants by inhibiting auxin-induced expression of the rolB gene. Plant Cell 8:477–487
Broeks A, Gerrard B, Allikmets R, dean M, Plasterk RH (1996) Homologues of the human multidrug resistance genes MRP and MDR contribute to heavy metal resistance in the soil nematode Caenorhabditis elegans. EMBO J 15:6132–6143
Capone I, Spanó L, Cardarelli M, Bellincampi D, Petit A, Costantino P (1989) Induction and growth properties of carrot roots with different complements of Agrobacterium rhizogenes T-DNA. Plant Mol Biol 13:43–52
Cardarelli M, Mariotti D, Pomponi M, Spanó L, Capone I, Costantino P (1987) Agrobacterium rhizogenes T-DNA genes capable of inducing hairy root phenotype. Mol Gen Genet 209:475–480
Cecchetti V, Pomponi M, Altamura MM, Pezzotti M, Marsilio S, D’Angeli S, Tornielli GB, Costantino P, Cardarelli M (2004) Expression of rolB in tobacco flowers affects the coordinated processes of anther dehiscence and style elongation. Plant J 38:512–525
Clemens S, Kim EJ, Neumann D, Schroeder JL (1999) Tolerance to toxic metals by a gene family of phytochelatin synthases from plants and yeast. EMBO J 18:3325–3333
Clemens S, Schroeder JI, Degenkolb T (2001) Caenorhabditis elegans expresses a functional phytochelatin synthase. Eur J Biochem 268:3640–3643
Cobbett CS (1999) A family of phytochelatin synthase genes from plant, fungal and animal species. Trends Plant Sci 4:335–337
Cobbett CS (2000) Phytochelatins and their roles in heavy metal detoxification. Plant Physiol 123:825–833
Connolly EL, Fett JP, Guerinot ML (2002) Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation. Plant Cell 14:1347–1357
Cunningham SD, Berti WR, Huang JWW (1995) Phytoremediation of contaminated soils. Trends Biotechnol 13:393–397
Curie C, Alonso JM, Le Jean M, Ecker JR, Briat JF (2000) Involvement of NRAMP1 from Arabidopsis thaliana in iron transport. Biochem J 3:749–755
De Knecht JA, Van Dillen M, Koevoets PLM, Schat H, Verkleji JAC, Ernst WHO (1994) Phytochelatins in cadmium-sensitive and cadmium-tolerant Silene vulgaris: chain length distribution and sulphide incorporation. Plant Physiol 104:255–261
Gisbert C, Ros R, De Haro A, Walker DJ, Bernal MP, Serrano R, Navarro-Aviñó J (2003) A plant genetically modified that accumulates Pb is especially promising for phytoremediation. Biochem Biophys Res Commun 303:440–445
Glaeser H, Coblenz A, Kruczek R, Ruttke I, Ebert-Jung A, Wolf K (1991) Glutathione metabolism and heavy metal detoxification in Schizosaccharomyces pombe. Isolation and characterisation of glutathione-deficent cadmium-sensitive mutants. Curr Genet 19:207–213
Gong J-M, Lee DA, Schroeder JI (2003) Long-distance root-to-shoot transport of phytochelatins and cadmium in Arabidopsis. Proc Natl Acad Sci USA 100:10118–10123
Grill E, Winnacker E-L, Zenk MH (1985) Phytochelatins: the principal heavy-metal complexing peptides of higher plants. Science 230:674–676
Grill E, Loffler S, Winnacker E-L, Zenk MH (1989) Phytochelatins, the heavy metal-binding peptides of plants, are synthesised from glutathione by a specific γ-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase). Proc Natl Acad Sci USA 86:6838–6842
Guerinot ML (2000) The ZIP family of metal transporters. Biochim Biophys Acta 1465:190–198
Guo DS, Xi YY, Wang AY, Zhang J, Yuan XY (1999) Contribution of an auxin to the uptake of nickel and cadmium in maize seedlings. Biomed Environ Sci 12:170–176
Howden R, Goldsbrough PB, Anderson CR, Cobbett CS (1995) Cadmium-sensitive, cad1 mutants of Arabidopsis thaliana are phytochelatin deficient. Plant Physiol 107:1059–1066
Kubota H, Sato K, Yamada T, Maitani T (2000) Phytochelatin homologs induced in hairy roots of horseradish. Phytochemistry 53:239–245
Lee S, Moon JS, Ko T, Petros D, Golsbrough PB, Korban SS (2003) Overexpression of Arabidopsis phytochelatin synthase paradoxically leads to hypersensitivity to cadmium stress. Plant Physiol 131:656–663
Li Y, Dhankher OM, Carreira L, Lee D, Chen A, Schroeder JI, Balish RS, Meagher RB (2004) Overexpression of phytochelatin synthase in Arabidopsis leads to enhanced arsenic tolerance and cadmium hypersensitivity. Plant Cell Physiol 45:1787–1797
Maliga PA, Sz-Breznovits A, Marton L (1973) Streptomycin-resistant plants from callus culture of haploid tobacco. Nat New Biol 244:29–30
Maurel C, Leblanc N, Barbier-Brigoo H, Perrot-Rochemann C, Bouvier-Durand M, Guern J (1994) Alteration of auxin perception in rolB-transformed tobacco protoplasts. Plant Physiol 105:1209–1215
Nedelkoska TV, Doran PM (2000) Hyperaccumulation of cadmium by hairy roots of Thlaspi caerulescens. Biotechnol Bioeng 67:607–615
Ortiz DF, Kreppel L, Speiser DM, Scheel G, McDonald G, Ow DW (1992) Heavy-metal tolerance in the fission yeast requires an ATP-binding cassette-type vacuolar membrane transporter. EMBO J 11:3491–3499
Papi M, Sabatini S, Bouchez D, Camilleri C, Costantino P, Vittorioso P (2000) Identification and disruption of an Arabidopsis zinc finger gene controlling seed germination. Genes Dev 1:28–33
Rauser WE (1990) Phytochelatins. Annu Rev Biochem 59:61–86
Salt DE, Rauser WE (1995) MgATP-dependent transport of phytochelatins across the tonoplast of oat roots. Plant Physiol 107:1293–1301
Salt DE, Prince RC, Pickering IJ, Raskin I (1995) Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiol 109:1427–1433
Salt DE, Smith RD, Raskin I (1998) Phytoremediation. Annu Rev Plant Physiol Plant Mol Biol 49:643–668
Sanitá di Toppi L, Lambardi M, Pecchioni N, Pazzagli L, Durante M, Gabrielli R (1999) Effects of cadmium stress on hairy roots of Daucus carota. J Plant Physiol 154:385–391
Sanitá di Toppi L, Prasad MNV, Ottonello S (2002) Metal chelating peptides and proteins in plants. In: Prasad MNV, Strzaka K (eds) Physiology and biochemistry of heavy metal detoxification and tolerance in plants. Kluwer, Dordrecht, pp 59–93
Sauge-Merle S, Cuiné S, Carrier P, Lecomte-Pradines C, Luu DT, Peltier G (2003) Enhanced toxic metal accumulation in engineered bacterial cells expressing Arabidopsis thaliana phytochelatin synthase. Appl Environ Microbiol 69:490–494
Spanó L, Mariotti D, Cardarelli M, Branca C, Costantino P (1988) Morphogenesis and auxin sensitivity of transgenic tobacco with different complements of Ri T-DNA. Plant Physiol 87:479–483
Spena A, Schmulling T, Koncz C, Schell J (1987) Independent and synergistic activity of rolA, B, and C loci in stimulating abnormal growth in plants. EMBO J 6:3891–3899
Thomine S, Wang R, Ward JM, Crawford NM, Schoereder JI (2000) Cadmium and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. Proc Natl Acad Sci USA 97:4991–4996
Vatamaniuk OK, Mari S, Lu Y, Rea PA (2000) Mechanism of heavy metal ion activation of phytochelatin (PC) synthase. Biol Chem 275:31451–31459
Vernoux T, Wilson RC, Seeley KA, Reichheld J, Muroy S, Brown S, Maughan SC, Cobbett CS, Van Montagu M, Inzè D, May MJ, Sung ZR (2000) The root meristemless1/cadmium sensitive2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell 12:97–109
Vögeli-Lange R, Wagner GJ (1996) Relationship between cadmium, glutathione and cadmium-binding peptides (phytochelatins) in leaves of intact tobacco seedlings. Plant Sci 114:11–18
Williams LE, Pittman JK, Hall JL (2000) Emerging mechanism for heavy metal transport in plants. Biochim Biophys Acta 1465:104–126
Yamaguchi H, Nishizawa NK, Nakanishi H, Mori S (2002) IDI7, a new iron-regulated ABC transporter from barley roots, localizes to the tonoplast. J Exp Bot 53:727–735
Zhu YL, Pilon-Smits EAH, Tarun AS, Weber SU, Jouanin L, Terry N (1999) Cadmium tolerance and accumulation in Indian mustard is enhanced by overexpressing γ-glutamylcysteine synthetase. Plant Physiol 121:1169–1177
Acknowledgements
Special thanks are given to Dr. Annette Pickford for helpful comments during manuscript revision. We thank Dr. Adele Figliolia (INP Rome, Italy) for helpful discussions, Prof. Rita Biasi and Dr. Patricia Gutierrez (University of Viterbo, Italy) for their help in ANOVA analysis. This work was partially supported by grants from Istituto Pasteur Fondazione Cenci-Bolognetti, and MIUR (FIRB, PRIN, Centro di Eccellenza in Biologia e Medicina Molecolare).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pomponi, M., Censi, V., Di Girolamo, V. et al. Overexpression of Arabidopsis phytochelatin synthase in tobacco plants enhances Cd2+ tolerance and accumulation but not translocation to the shoot. Planta 223, 180–190 (2006). https://doi.org/10.1007/s00425-005-0073-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-005-0073-3