Abstract
Synthetic biological systems are becoming more and more feasible for commercial and medical purposes through the genetic engineering of several components. The simple assembly of a genetic circuit was shown to stimulate the removal of copper by bacteria through the engineering of a two-component system. The CusSR two-component systems is a regulator of Escherichia coli copper homeostatic system. In this system, genetic circuits of CusSR were fused to a cell surface display system for metal adsorption; this system is suitable for the display of a copper binding peptide through outer membrane protein C (OmpC). E. coli ompC codes for an outer membrane pore protein (porin) are induced at high osmolarity and temperature, which can also be used as an anchoring motif to accept the passenger proteins. The bacteria that produce the chimeric OmpC containing the copper binding peptide adsorbed maximum concentrations of 92.2 μmol of Cu2+/gram dry weight of bacterial cells. This synthetic bacterial system senses the specific heavy metal and activates a cell surface display system that acts to remove the metal.
Similar content being viewed by others
Introduction
As a result of industrialization, heavy metal pollution has become a serious problem in both developing and developed countries throughout the world. In order to solve this problem, a variety of expression systems promoting the display of chimeric proteins on the surfaces of microbial cells have emerged for the bioremediation of heavy metals [1, 2]. Cell surface display technology has made possible a wide range of applications in the biotechnological and industrial fields, such as the recovery of harmful chemicals and heavy metals, live-vaccine development, and screening of peptide libraries without causing metabolic abnormalities in the host system [3–6]. This technology allow for the expressions of proteins or peptides on the surfaces of the cells using the surface proteins of bacteria, yeast, or even mammalian cells as anchoring motifs [7–9].
Copper can be extremely toxic to microbes and thus necessitates homeostatic mechanisms for cell survival [4, 10, 11]. Copper induces transcription of both copA and cueO, which encode for the copper transporting ATPase and periplasmic multicopper oxidase, respectively, leading to the detoxification of copper [12, 13]. At higher concentrations of Cu2+, the system described above fails to exogenously efflux and sense the metal. However, the two-component system (TCS) CusSR allows for exogenous copper detection via membrane-associated sensor kinase. The response regulator in CusSR is involved in the transcription of copper and silver efflux systems under anaerobic conditions and is utilized in aerobic conditions under extreme copper stress [10, 14]. In response to high Cu2+ concentrations, the CusSR system present in the bacteria specifically activates the expressions of CusCFBA genes. The CusC is an outer membrane protein that undergoes specific acquisition of tolerance under high Cu2+ and Ag2+ concentrations [15]. Therefore, mechanistic path from copper stimulus to regulation of CusCFBA genes depends on a number of characteristics of the TCS. By manipulating these characteristics, we can create sensor kinases that respond to new signals, promoters that respond to different response regulators or actuators. Currently, the main application of bacterial two-component system is its use as a sensor for chemical and biological components in their surrounding enviornment by altering actuators (reporters), but in the near future, TCS can be exploited for the design of complex synthetic network suitable for commercial or medical application.
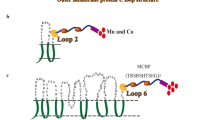
A number of outer membrane proteins are now in use for the display on the cell surface and action of enzymes, peptide libraries, antigenic determinants, or single-chain antibodies on the surface of gram negative bacteria [16]. Esherichia coli OmpC, one of the most abundant and well characterized. OmpC molecule consists of 16 transmembrane, antiparallel β-strands, which produce a β-barrel structure surrounding a large channel and are connected by seven internal loops and eight external loops [17]. In general, the amino acid sequences of the external loops are less conservative, and thus, these loops may be relatively tolerant to insertion and deletion. Therefore, we decided to use C-terminal deletion–fusion strategy on the external loops as the point of insertion for foreign peptides for cell surface display. In this study, we took the initiative in broadening the application of TCS by investigating the display of a copper binding peptide (CBP) on the surface of E. coli using manipulated actuators OmpC as an anchoring motif in response to exogenous Cu2+ in the two-component CusSR signal transduction system (Fig. 1). The dynamic characteristic of cusC gene expression via exogenous Cu2+ was studied, and a simple copper adsorption system was constructed to adsorb exogenous Cu2+ on the bacterial surface. These engineered bacteria behave like normal bacteria until it senses copper. If this bacteria senses copper, they activate genetic circuit, display peptide, and remove copper automatically
Engineered E. coli to detect and remove copper. Periplasmic metal sensing receptors detect Cu2+, which phosphorylate the histidine kinase domain and response regulator from the OmpR family of TCS in the receptor. This activates the synthetic genetic circuit of CusSR TCS, resulting in a Cu2+-binding peptide on the E. coli surface available for bioadsorption
Materials and Methods
Bacterial Strains and Media
E. coli XL1-Blue was used as a host strain for all recombinant DNA manipulation. All recombinant E. coli were cultivated in Luria–Bertani (LB) medium (10 g/l bacto-tryptone, 5 g/l bacto-yeast extract, and 5 g/l NaCl) supplemented with 100 μg/ml of ampicillin at 37 °C with vigorous shaking (200 rpm) unless otherwise noted.
Construction of the Plasmid for the Copper Removal System
The genomic region containing the 156-bp cusC–cusR intergenic region was amplified from E. coli genomic DNA with oligonucleotides cusC_FEcoRI and cusC_RsacI (Table 1). The polymerase chain reaction (PCR) was performed with the MJ Mini personal thermal cycler (BioRad Laboratories, USA) using the Expand High Fidelity PCR System (Roche Molecular Biochemicals, Mannheim, Germany). The PCR products were then digested with EcoRI and SacI and were ligated with pUC19 to construct the pUCup1 plasmid [18]. E. coli ompC genes were amplified from the genomic DNA of E. coli using primers that were designed based on reported genome sequences [19]. The truncated, metal-binding peptide-containing E. coli outer membrane protein C (ompC t ) were integrated using the C-terminal deletion–fusion strategy [17, 20]. For the expression of CBP (SPHHGGW) on the cell surface [21], the ompC t genes encoding the 331 amino acids from the N terminus were amplified using two complimentary pairs of oligonucleotides, as shown in Table 1. The PCR product was cloned into the pUCup1 plasmid using SacI and KpnI to construct pCC1056, in which the chimeric protein was under the control of a cusC promoter (Fig. 2). This plasmid was transformed into chemically competent E. coli cells for further studies.
Evaluation of Engineered Bacteria in Response to Copper
A single colony of E. coli harboring pCC1056 was grown overnight in nutrient-rich LB medium supplemented with 100 μg/ml ampicillin and was incubated at 37 °C in an orbital shaker at 200 rpm until reaching an optical density of 0.5 at 600 nm (OD600). Then the cells were grown for an additional 4 h in the presence of varying concentrations of CuSO4 to evaluate the dynamics of the CusSR TCS.
The transcriptional activities of the cusC gene in response to Cu2+ in the E. coli cells harboring pCC1056 were measured using real-time quantitative PCR (RT-PCR). A metal–bacteria mixture was obtained using the protocol described above. After 4 h, the cells were then harvested via centrifugation for total RNA preparation using the RNeasy Mini kit according to the manufacturer’s instruction (Qiagen), which was then followed by DNase treatment. Reverse transcription was performed with a complementary DNA (cDNA) synthesis kit (Applied Biosystems) using a random hexamer primer mix according to the manufacturer’s instructions. Specific primers were designed with OLIGO software (version 5.0; Molecular Biology Insights, Cascade, CO, USA) for the quantitative expression of the cusC gene (Table 1). Samples for which the RT step was omitted were used as negative controls to confirm that the extracted RNA was not contaminated with DNA. RT-PCR reactions were performed on the Mini-opticon detection system with the SYBR Green PCR Master mix, which was used as recommended by the manufacturer. Each quantitative RT-PCR experiment was performed on three replicates of biological samples consisting of separate cultures grown under identical conditions (n = 3), and the results were calculated automatically using Mini-opticon software with 16s RNA as an internal control [22].
After 4 h in the presence of 0.5 mM concentrations of CuSO4, the cells were then harvested. Protein samples prepared and were analyzed by 12% (w/v) sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) as described by Laemmli [23]. Fractionated protein samples were stained with Coomassie brilliant blue R-250 (Bio-Rad Laboratories, Hercules, CA, USA).
Evaluation of Metal Bioadsorption by the Engineered Bacteria
E. coli strains harboring pCC1056 were grown separately overnight at 37 °C. The overnight culture was diluted 100-fold in fresh LB medium supplemented with 100 μg/ml ampicillin and incubated at 37 °C in an orbital shaker at 250 rpm. When the OD600 of the cultures reached 0.5, the cells were inducted with varying concentration of 0.5 and 1.0 mM CuSO4 for 4 h. Prior to determination of metal content, bacterial cells were cultivated to the stationary growth phase in LB medium and were washed twice with 0.85% (w/v) NaCl. They were then treated with 5 mM EDTA on ice for 30 min to remove the cell surface-bound Cu2+, and the resulting concentration of Cu2+ in the supernatant was measured directly using inductively coupled plasma–mass spectrometry (HP 4500, Yokogawa, Tokyo, Japan). The same procedure was used to determine bioadsorption of Zn2+ with E. coli harboring pCC1056.
Results and Discussion
Evaluation of Dynamic Characteristics of cusC
The bacterial copper adsorption system constructed through integration of chimeric OmpC and cusC promoter (P cusC ) to respond to and adsorb exogenous copper. To determine whether the response of Cu2+ concentration on engineered bacteria activated expression of the cusC gene by the CusSR TCS, the dynamics of CusSR TCS was studied using quantitative RT-PCR. In the presence of an exogenous copper concentration, CusSR TCS activates the expression of the cusC gene [10]. The RNA samples from E. coli cultures grown for 4 h in LB media supplemented with varying concentrations of Cu2+ were used, and the results are shown in Fig. 3A. Our findings suggested that the transcript level of the cusC gene is significantly increased in cells grown at elevated Cu2+ concentration in LB medium. At a 0.1 mM induction, the increases in the transcriptional levels of cusC were not significant (P < 0.05, n = 3) compared to those of the control; however, with an induction of 1 mM of copper, a significant increase of approximately sixfold (P < 0.05, n = 3) was observed in the LB media. The results revealed that the presence of copper ions in the nutrient-rich medium induced the transcriptional level of cusC gene to higher concentrations compared to those of the control.
A Transcriptional levels of the cusC gene in LB medium after 4 h of Cu2+ exposure. The C t value was normalized to the mean C t value of the 16s RNA as an internal control. B Effect of cells harboring pUC19 and pCC1056 on copper tolerance. Growth curves with varying concentration of CuSO4 are shown. Overnight cultures were diluted 100-fold in fresh LB medium supplemented with 100 μg/ml ampicillin, and after 2 h incubatation at 37 °C, indicated concentration of CuSO4 were added. Cell growth was monitored as the optical density at 600 nm after 4 h of incubation at 37 °C
Evaluation of Copper Bioadsorption
The results presented above demonstrate that the CusSR TCS of the bacteria shows a dynamic response upon exposure to exogenous Cu2+ concentrations. As a result, a cell surface bacterial system was constructed to assist in the selective adsorption of exogenous Cu2+. Here, the OmpC coding region was integrated to heterologous peptides (CBPs), which have already been shown to have a high affinity for Cu2+ ions. A synthetic DNA fragment encoding this chimeric gene was inserted into the pUCup1 to construct pCC1056, in which the chimeric protein was under the control of a cusC promoter (Fig. 2). Localization of target polypepetides (OmpC-CBP) from the harvested recombinant E. coli XL1-Blue harboring pCC1056 was determined by SDS-PAGE, indicating that ompC-CBP was expressed under the control of cusC promoter in the presence of exogenous copper. Figure 4B shows the SDS-PAGE of OmpC-CBP fusion protein from recombinant E. coli harboring pCC1056.
A Bioadsorption of Cu2+ by E. coli (pCC1056). The metal contents of these samples were determined using inductively coupled plasma–mass spectrometry. The data are aggregate results from replicate experiments (n = 3). a Uninduced cells, b 0.5 mM CuSO4 induced, c 1.0 mM CuSO4 induced. B Localization of the OmpC-CBP fusion in protein extracts and culture medium of pCC1056 transformed XL1-Blue cells (2, 4, and 6) and non-induced recombinant plasmid as controls (1, 3, and 5). Overnight cultures were diluted 1/10 in fresh medium (20 ml) containing 100 mg/ml ampicillin, induced after 2 h with 0.5 mM CuSO4, and grown for a further 4 h. The culture medium was subsequently recovered for protein samples; proteins were detected in Coomassie Blue-stained SDS-PAGE gels. The location of the OmpC-CBP fusion protein is indicated by arrows
The ability of these engineered bacteria to absorb heavy metals was examined to determine the effect of displaying the CBP. Under these experimental conditions, E. coli cells that produced the chimeric ompC t -CBP proteins were cultured, and the wild-type E. coli cells with no chimeric protein and only ompC without promoter and metal binding protein served as a control. The cells harboring only OmpC showed identical values with the wild-type E. coli cells harboring pUC19 (data not shown). When cultures reached an OD600 of 0.5, the cells were induced when exposed to 0.5 and 1.0 mM CuSO4. We also found slow growth rate in cells harboring pCC1056 compared to cells harboring pUC19 due to copper ion induction and cell surface display system (Fig. 3B). As shown in Fig. 4A, cells harboring pCC1056 could adsorb 92.2 and 78.7 μmol of Cu2+/gram DCW when induced with 0.5 and 1 mM CuSO4, respectively, while the uninduced cell strain harboring pCC1056 adsorbed 18.8 μmol of Cu2+/gram DCW.
A slight increase in the Cu2+ adsorption by uninduced cells was observed due to leaking expression of ompC t -CBP [24]. Therefore, cells displaying ompC t -CBP at 0.5 and 1 mM CuSO4 induction adsorbed Cu2+ at ~5 and ~4 times higher levels, respectively, than did the cells not displaying ompC t -CBP (control plasmid). The 0.5 mM CuSO4 induced cell showed higher Cu2+ adsorption than 1.0 mM induced cell due to the toxicity of copper. Induction with CuSO4 concentrations >1 mM was found to decrease cell growth by more than 50%. This is due to the saturation of copper homeostasis systems, which renders them unable to detoxify excess Cu2+ in the environment. When low concentrations of CuSO4 (0.01 or 0.1 mM) were used for induction, Cu2+ adsorbed <10% of the maximum Cu2+ adsorption (data not shown). The CusSR system was not activated by low concentration of CuSO4, and only the Cue system was activated to detoxify Cu2+ [25, 26]. Another possible mechanism in the decrease in copper adsorption by the cells harboring pCC1056 was copper accumulated into cell by OmpC-CBP fusion protein that fails in translocation into cell membrane.
Regarding the adsorption of other metals, namely, Zn2+, by induced cells, the amount recovered from the cell surface was found to be lower than that of Cu2+ due to the selective metal adsorption peptide on the cell surface (Fig. 4A).
Conclusion
In the present study, E. coli strain was engineered to sense and remove copper through integration of the copper sensing TCS and cell surface display of OmpCt-CBP chimera protein. The engineered bacteria selectively adsorbed Cu2+ in rich LB medium, and this result indicated that the engineered E. coli system may be used to sense and remove copper contamination in many locations such as soil, groundwater, or industrial surfaces. The strategy employed in this study can be applied for the development of other heavy metal and contaminant removing system.
Abbreviations
- TCS:
-
Two component system
References
Dong, J., Liu, C., Zhang, J., Xin, Z. T., Yang, G., Gao, B., et al. (2006). Chemical Biology & Drug Design, 68, 107–112.
Narita, J., Okano, K., Tateno, T., Tanino, T., Sewaki, T., Sung, M. H., et al. (2006). Applied Microbiology and Biotechnology, 70, 564–572.
Bae, W., Wu, C. H., Kostal, J., Mulchandani, A., & Chen, W. (2003). Applied and Environmental Microbiology, 69, 3176–3180.
Harvey, B. R., Georgiou, G., Hayhurst, A., Jeong, K. J., Iverson, B. L., & Rogers, G. K. (2004). Proceedings of the National Academy of Sciences of the United States of America, 101, 9193–9198.
Lee, J.-S., Shin, K.-S., Pan, J.-G., & Kim, C.-J. (2000). Nature Biotechnology, 18, 645–648.
Taschner, S., Meinke, A., von Gabain, A., & Boyd, A. P. (2002). Biochemical Journal, 367, 393–402.
Cornelis, P. (2000). Current Opinion in Biotechnology, 11, 450–454.
Lee, S. Y., Choi, J. H., & Xu, Z. (2003). Trends in Biotechnology, 21, 45–52.
Stahl, S., & Uhlen, M. (1997). Trends in Biotechnology, 15, 185–192.
Munson, G. P., Lam, D. L., Outten, F. W., & O'Halloran, T. V. (2000). Journal of Bacteriology, 182, 5864–5871.
Rensing, C., & Grass, G. (2003). FEMS Microbiology Reviews, 27, 197–213.
Petersen, C., & Moller, L. B. (2000). Gene, 261, 289–298.
Stoyanov, J. V., Hobman, J. L., & Brown, N. L. (2001). Molecular Microbiology, 39, 502–511.
Grass, G., & Rensing, C. (2001). Journal of Bacteriology, 183, 2145–2147.
Franke, S., Grass, G., & Nies, D. H. (2001). Microbiology, 147, 965–972.
Georgiou, G., Poetschke, H. L., Stathopoulos, C., & Francisco, J. A. (1993). Trends in Biotechnology, 11, 6–10.
Xu, Z., & Lee, S. Y. (1999). Applied and Environmental Microbiology, 65, 5142–5147.
Sambrook, J., & Russell, D. W. (2001). Molecular cloning:a laboratory manual (3rd ed.). Cold Spring Harbor: Cold Spring Harbor Laboratory.
Blattner, F. R., Plunkett, G., 3rd, Bloch, C. A., Perna, N. T., Burland, V., Riley, M., et al. (1997). Science, 277, 1453–1462.
Jeanteur, D., Lakey, J. H., & Pattus, F. (1991). Molecular Microbiology, 5, 2153–2164.
Patwardhan, A. V., Goud, G. N., Koepsel, R. R., & Ataai, M. M. (1997). Journal of Chromatography, 787, 91–100.
Laemmli, U. K. (1970). Nature, 227, 680–685.
Eleaume, H., & Jabbouri, S. (2004). Journal of Microbiological Methods, 59, 363–370.
Kotrba, P., Doleckova, L., de Lorenzo, V., & Ruml, T. (1999). Applied and Environmental Microbiology, 65, 1092–1098.
Grass, G., & Rensing, C. (2001). Biochemical and Biophysical Research Communications, 286, 902–908.
Outten, F. W., Huffman, D. L., Hale, J. A., & O'Halloran, T. V. (2001). Journal of Biological Chemistry, 276, 30670–30677.
Acknowledgment
This work was supported by the Korean Systems Biology Research Project (20100002164) of the Ministry of Education, Science and Technology (MEST) through the National Research Foundation of Korea and by a Korea Research Foundation grant funded by the Korean Government (MOEHRD) (KRF-2007-211-D00026).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ravikumar, S., Yoo, Ik., Lee, S.Y. et al. Construction of Copper Removing Bacteria Through the Integration of Two-Component System and Cell Surface Display. Appl Biochem Biotechnol 165, 1674–1681 (2011). https://doi.org/10.1007/s12010-011-9386-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-011-9386-9