Abstract
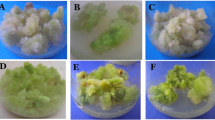
Efficient plantlet regeneration with and without intermediate callus phase was achieved for a selected genotype of Aloe vera L. which is sweet in test and used as a vegetable and source of food. Random amplified polymorphic DNA (RAPD) and inter simple sequence repeats (ISSR) marker assays were employed to evaluate genetic stability of plantlets and validate the most reliable method for true-to-type propagation of sweet aloe, among two regeneration systems developed so far. Despite phenotypic similarities in plantlets produced through both regeneration systems, the differences in genomic constituents of plantlets produced through intermediate callus phase using soft base of inflorescence have been effectively distinguished by RAPD and ISSR markers. No polymorphism was observed in regenerants produced following direct regeneration of axillary buds, whereas 80% and 73.3% of polymorphism were observed in RAPD and ISSR, respectively, in the regenerants produced indirectly from base of the inflorescence axis via an intermediate callus phase. Overall, 86.6% of variations were observed in the plantlets produced via an intermediate callus phase. The occurrence of genetic polymorphism is associated with choice of explants and method used for plantlet regeneration. This confirms that clonal propagation of sweet aloe using axillary shoot buds can be used for commercial exploitation of the selected genotype where a high degree of fidelity is an essential prerequisite. On the other hand, a high degree of variations were observed in plantlets obtained through indirect regeneration and thus cannot be used for the mass multiplication of the genotype; however, it can be used for crop improvement through induction of somaclonal variations and genetic manipulations.




Similar content being viewed by others
References
Campestrini, L. H., Kuhnen, S., Lemos, P. M. M., Bach, D. B., Dias, P. F., & Maraschin, M. (2006). Journal of Technology Management Innovation, 1, 77–79.
Roy, S. C., & Sarkar, A. (1991). Scientia Horticulturae, 47, 107–113.
Meyer, H. J., & van Staden, J. (1991). Plant Cell Tissue and Organ Culture, 26(3), 167–171. doi:10.1007/BF00039939.
Richwine, A. M., Tipton, J. L., & Thompson, A. (1995). Horticulturea Science, 30, 1443–1444.
Abrie, A. L., & van Staden, J. (2001). Plant Growth Regulation, 33, 19–23. doi:10.1023/A:1010725901900.
Liao, Z., Chen, M., Tan, F., Sun, X., & Tang, K. (2004). Plant Cell Tissue and Organ Culture, 76(1), 83–86. doi:10.1023/A:1025868515705.
Aggarwal, D., & Barna, K. S. (2004). Journal of Plant Biochemistry and Biotechnology, 13, 77–79.
Velcheva, M., Faltin, Z., Vardi, A., Eshdat, Y., & Perl, A. (2005). Plant Cell Tissue and Organ Culture, 83(3), 293–301. doi:10.1007/s11240-005-7192-1.
Bairu, M. W., Stirk, W. A., Dolezal, K., & van Staden, J. (2007). Plant Cell Tissue and Organ Culture, 90, 15–23. doi:10.1007/s11240-007-9233-4.
Singh, M., Rathore, M. S., Panwar, D., Rathore, J. S., Dagla, H. R., & Shekhawat, N. S. (2009). Journal of Sustainable Forestry, 28, 935–950. doi:10.1080/10549810903344660.
Rathore, M. S., Chikara, J., & Shekhawat, N. S. (2011). Applied Biochemistry and Biotechnology, 163, 860–868. doi:10.1007/s12010-010-9090-1.
Salvi, N. D., George, L., & Eapen, S. (2001). Plant Cell Tissue and Organ Culture, 66, 113–119.
Braun, A. C. (1959). Proceedings of National Academy of Sciences USA, 45, 932–938.
Bairu, M. W., Aremu, A. O., & Van Staden, J. (2011). Plant Growth Regulation, 63, 147–173.
Brar, D. S., & Jain, S. M. (1998). In S. M. Jain, D. S. Brar, & B. S. Ahloowalia (Eds.), Somaclonal variation and induced mutations in crop improvement (pp. 15–37). Dordrecht: Kluwer Academic.
Jain, S. M. (2001). Euphytica, 118, 153–166.
Bhatia, R., Singh, K. P., Jhang, T., & Sharma, T. R. (2009). Scientia Horticulturae, 119, 208–211.
Lu, G., Zhang, X., Zou, Y., Zou, Q., Xiang, X., & Cao, J. (2007). Plant Cell Tissue and Organ Culture, 88, 319–327.
Smykal, P., Valledor, L., Rodriguez, R., & Griga, M. (2007). Plant Cell Reports, 26, 1985–1998.
Martin, M., Sarmento, D., & Oliveira, M. M. (2004). Plant Cell Reports, 23, 492–496.
Palombi, M. A., & Damiano, C. (2002). Plant Cell Reports, 20, 1061–1066.
Murashige, T., & Skoog, F. (1962). Physiologia Plantarum, 15, 473–479. doi:10.1111/j.1399-3054.1962.tb08052.x.
Doyle, J. J., & Doyle, J. L. (1990). Focus, 12, 13–15.
Williams, J. G., Kubelik, A. R., Livak, K. J., Rafalski, J. A., & Tingey, S. V. (1990). Nucleic Acids Research, 18, 6531–6535.
Goto, S., Thakur, R. C., & Ishii, K. (1998). Plant Cell Reports, 8, 193–197.
Mallon, R., Rodrıguez-Oubina, J., & Gonzalez, M. L. (2010). Plant Cell Tissue and Organ Culture, 101, 31–39.
Ray, T., Dutta, I., Saha, P., Das, S., & Roy, S. C. (2006). Plant Cell Tissue and Organ Culture, 85, 11–21.
Al-Zahim, M. A., Ford-Lloyd, B. V., & Newbury, H. J. (1999). Plant Cell Reports, 18, 473–477.
Acknowledgments
The authors are thankful to the Council of Scientific and Industrial Research (CSIR) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rathore, M.S., Chikara, J., Mastan, S.G. et al. Assessment of Genetic Stability and Instability of Tissue Culture-Propagated Plantlets of Aloe vera L. by RAPD and ISSR Markers. Appl Biochem Biotechnol 165, 1356–1365 (2011). https://doi.org/10.1007/s12010-011-9352-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-011-9352-6