Abstract
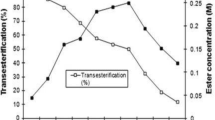
Kinetic resolution of (R,S)-2-butanol using enzymatic synthesis of esters has been studied. (R,S)-2-Butanol is commonly found as a racemic mixture, and the products of its esterification are racemic mixtures too. This work is of great significance in the field of the enzymatic kinetic resolution due to the little information found in literature about the resolution of (R,S)-2-butanol as pure compound. So, this article is a contribution about the enzymatic resolution of (R,S)-2-butanol. The reaction here studied is the esterification/transesterification of (R,S)-2-butanol in organic media (n-hexane) using as biocatalyst the lipase Novozym 435®. The main target of this study is to analyze the influence of certain variables in this reaction. Some of these variables are acyl donor (acids and esters), concentration of substrates, enzyme/substrate ratio, and temperature. The main conclusions of this study are the positive effect of higher substrates concentration (1.5 M) and larger amount of enzyme (13.8 g mol−1 substrate) on kinetic resolution rate but not a very noticeable effect on enantiomeric excesses. The longer the carboxylic acid chain is, the better results are obtained. Besides to achieve a satisfactory kinetic resolution, it is recommendable to select reaction times (180 min) at which the highest substrate enantiomeric excess is reached (~60%). The temperature has not an appreciable influence on the resolution in the range studied (40–60 °C). When an ester (vinyl acetate) is used as acyl donor, the resolution shows better results than when using a carboxylic acid as acyl donor (ees ~ 90% at 90 min). Moreover, Michaelis–Menten parameters, v max and K M, were determined, 0.04 mol 1−1 min−1 and 0.41 mol 1−1, respectively.










Similar content being viewed by others
References
Kumar, I., Manju, K., & Jolly, R. S. (2001). Tetrahedron-Asymmetry, 12, 1431–1434.
Kasprzyk-Hordern, B. (2010). Chemical Society Reviews, 39, 4466–4503.
Fogassy, E., Nógrádi, M., Kozma, D., Egri, G., Pálovics, E., & Kiss, V. (2006). Organic and Biomolecular Chemistry, 4, 3011–3030.
Fieser, L. F. (1966). Basic organic chemistry. Tokyo: Maruzen.
Zaugg, H. E. (1955). Journal of the American Chemical Society, 77(10), 2910.
Fujima, Y., Ikunaka, M., Inoue, T., & Matsumoto, J. (2006). Organic Process Research and Development, 10(5), 905–913.
Wang, Y., Wang, R., Li, Q., Zhang, Z., & Feng, Y. (2009). Journal Molecule Catalysis B: Enzyme, 56, 142–145.
Patel, R. N. (2008). Coordination Chemistry Reviews, 252, 659–701.
Romero, M. D., Calvo, L., Alba, C., Habulin, M., Primozic, M., & Knez, Ž. (2005). Journal Supercritical Fluids, 33, 77–84.
Znez, Ž. (2009). Journal Supercritical Fluids, 47, 357–372.
Yasmin, T., Jiang, T., Han, B., Zhang, J., & Ma, X. (2006). Journal Supercritical Fluids, 41, 27–31.
De los Ríos, A. P., Hernández-Fernández, F. J., Tomás-Alonso, F., Gómez, D., & Víllora, G. (2008). Process Biochemistry, 43, 892–895.
Fan, Y., & Qian, J. (2010). Journal Molecule Catalysis B: Enzyme, 66, 1–7.
Lozano, P. (2010). Green Chemistry, 12, 555–569.
Romero, M. D., Calvo, L., Alba, C., & Daneshfar, A. (2007). Journal of Biotechnology, 127, 269–277.
Habulin, M., & Knez, Ž. (2009). Journal Molecule Catalysis B: Enzyme, 58, 24–28.
An, I., Onyeozili, E. N., & Maleczka, R. E., Jr. (2010). Tetrahedron-Asymmetry, 21, 527–534.
Reigada, J. B., Tcacenco, C. M., Andrade, L. H., Kato, M. J., Porto, A., & Lago, J. H. (2007). Tetrahedron-Asymmetry, 18, 1054–1058.
Gotor-Fernández, V., Busto, E., & Gotor, V. (2006). Advanced Synthesis and Catalysis, 348, 797–812.
Briggs, G. E., & Haldane, J. B. (1925). Biochemical Journal, 19, 338–339.
Rotticci, D., Hæffner, F., Orrenius, C., Norin, T., & Hult, K. (1998). Journal Molecule Catalysis B: Enzyme, 5, 267–272.
Romero, M. D., Calvo, L., Alba, C., Daneshfar, A., & Ghaziaskar, H. S. (2005). Enzyme and Microbial Technology, 37(1), 42–48.
Ottosson, J., & Hult, K. (2001). Journal Molecule Catalysis B: Enzyme, 11, 1025–1028.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Romero, M.D., Gomez, J.M., Diaz-Suelto, B. et al. Kinetic Resolution of (R,S)-2-Butanol Using Enzymatic Synthesis of Esters. Appl Biochem Biotechnol 165, 1129–1140 (2011). https://doi.org/10.1007/s12010-011-9330-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-011-9330-z