Abstract
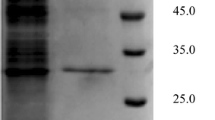
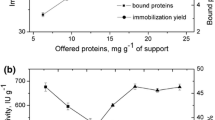
Microorganisms producing lipase were isolated from soil and sewage samples and screened for enantioselective resolution of (R,S)-methyl mandelate to (R)-mandelic acid. A strain designated as GXU56 was obtained and identified as Burkholderia sp. Preparing immobilized GXU56 lipase by simple adsorption on octyl sepharose CL-4B, the optimum temperature was shifted from 40 °C (free lipase) to 50 °C (immobilized lipase), and the optimum pH was shifted from 8.0 (free lipase) to 7.2 (immobilized lipase). The immobilized enzyme displayed excellent stability in the pH range of 5.0–8.0, at the temperatures below 50 °C and in organic solvents compared with free enzyme. Enantioselectivity ratio for (R)-mandelic acid (E) was dramatically improved from 29.2 to more than 300 by applying immobilized lipase in the resolution of (R,S)-methyl mandelate. After five cycles of use of immobilized lipase, conversion and enantiomeric excess of (R)-mandelic acid were 34.5% and 98.5%, respectively, with enantioselectivity ratio for (R)-mandelic acid (E) of 230. Thus, octyl-sepharose-immobilized GXU56 lipase can be used as a bio-resolution reagent for producing (R)-mandelic acid.




Similar content being viewed by others
References
Kazushi, K., Kenichi, S., Yukihiko, H., Hiroyuki, N., & Kazuhiko, S. (1996). Design of resolving reagents: p-Substituted Mandelic Acid as Resolving Reagents for 1-Arylalkylamines. Tetrahedron: Asymmetry,7,1539–1542.
Savidge, T.A. (1984). Enzymatic conversions used in the production of penicillins and cephalosporins. pp: 177−224. In: Biotechnology of Industrial Antibiotics, Vandamme, Erick. J. (ed.), Marcel Dekker, New York.
Mills, J., Schmiegal, K. K., & Sham, W. N. (1983). Phenethanolamines compositions containing the sane, and method for effecting weight control. US Patent 4,391,826. US Patent, 4, 391,826.
Arai, T., Koike, H., Hirata, K., & Oizumi, H. (1988). Separation of pyridone carboxylic acid enantiomers by high-performance liquid chromatography using copper(ІІ)-L-amino acid as the eluent. Journal of Chromatography, 448, 439–444.
Daniela, S., Juan, A. C., Anna, M. G., Silvia, C., Remo, B., Cristina, P., & Manuel, V. (2002). Liquid membranes for chiral separations. Application of cinchonidine as a chiral carrier. Journal of Separation Science, 25, 229–238.
Byung-Yong, K., Ki-Chul, H., Hee-Sang, S., Namhyun, C., & Won-Gi, B. (2000). Optical resolution of (R,S)-(±)-mandelic acid by Pseudomonas sp. . Biotechnology Letters, 22, 1871–1875.
Ganapati, D. Y., & Sivakumar, P. (2004). Enzyme-catalysed optical resolution of mandelic acid via (R,S)-(±)-methyl mandelate in non–aqueous media. Biochemical Engineering Journal, 19, 101–107.
Praveen, K., Anirban, B., Mayilraj, S., & Uttam, C. B. (2004). Screening for enantioselective nitrilases: kinetic resolution of racemic mandelonitrile to (R)-(-)-mandelic acid by new bacterial isolates. Tetrahedron: Asymmetry, 15, 207–211.
Cristiane, P., & Maria, G. N. (2006). Effects of organic solvents and ionic liquids on the aminolysis of (R,S)-methyl mandelate catalyzed by lipases. Tetrahedron: Asymmetry, 17, 428–433.
Neide, Q., & Maria, D. G. N. (2002). Pseudomonas sp. lipase immobilized in polymers versus the use of free enzyme in the resolution of (R,S)-methyl mandelate. Tetrahedron Letters, 43, 5225–5227.
Rohit, S., Yusuf, C., & Uttam, C. B. (2001). Production, purification, characterization, and applications of lipases. Biotechnol Advances, 19, 627–662.
Fernandez-Lorente, G., Fernández-Lafuente, R., Palomo, J. M., Mateo, C., Bastida, A., Coca, J., et al. (2001). Biocatalyst engineering exerts a dramatic effect on selectivity of hydrolysis catalyzed by immobilized lipases in aqueous medium. Journal of Molecular Catalysis B, Enzymatic, 11, 649–656.
Chaubey, A., Parshad, R., Koul, S., Taneja, S. C., & Qazi, G. N. (2006). Arthrobacter sp. lipase immobilization for improvement in stability and enantioselevtivity. Applied Microbiology and Biotechnology, 73, 598–606.
Dong-Woo, L., You-Seok, K., Ki-Jun, K., Byung-Chan, K., Hak-Jong, C., Doo-Sik, K., et al. (1999). Isolation and characterization of a thermophilic lipase from Bacillus thermoleovorans ID-1. FEMS Microbiology Letters, 179, 393–400.
Mohammad, H., Kathryn, M. U. B., Szilvia, M. Z., Naoki, M., Yoshinobu, N., Isao, Y., et al. (2004). Isolation, Identification, and Characterization of a Novel, Oil-Degrading Bacterium, Pseudomonas aeruginosa T1. Current Microbiolgy, 49, 108–114.
Jiangke, Y., Daoyi, G., & Yunjun, Y. (2007). Cloning, expression and characterization of a novel thermal stable and short-chain alcohol tolerant lipase from Burkholderia cepacia G63. Journal of Molecular Catalysis B, Enzymatic, 45, 91–96.
Ryo, I., Manabu, S., & Fusako, K. (2001). Screening and characterization of trehalose-oleate hydrolyzing lipase. FEMS Microbiology Letters, 195, 231–235.
Bruno, L. M., Coelho, J. S., Melo, E. H. M., & Lima-Filho, J. L. (2005). Characterization of Mucor miehei lipase immobilized on polysiloxane-polyvinyl alcohol magnetic particles. World Journal of Microbiology and Biotechnology, 21, 189–192.
Jose, M. P., Gloria, F. L., Cesar, M., Claudia, O., Roberto, F. L., & Jose, M. G. (2002). Modulation of the enantioselectivity of lipases via controlled immobilization and medium engineering: hydrolytic resolution of mandelic acid esters. Enzyme and Microbial Technology, 31, 775–783.
Roberto, F. L., Pilar, A., Pilar, S., Gloria, F. L., & José, M. G. (1998). Immobilization of lipases by selective adsorption on hydrophobic supports. Chemistry and Physics of Lipids, 93, 185–197.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wei, HN., Wu, B. Screening and Immobilization Burkholderia sp. GXU56 Lipase for Enantioselective Resolution of (R,S)-Methyl Mandelate. Appl Biochem Biotechnol 149, 79–88 (2008). https://doi.org/10.1007/s12010-007-8125-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-007-8125-8