Abstract
Background
The concept of osseointegration involves direct contact between titanium implant and bone. This transcutaneous prosthetic system for amputees is intended to assure stable long-term fixation. Most metal transcutaneous implants have failed, primarily owing to infection.
Questions/purposes
We determined the frequency and describe the presentation of infectious complications with this novel method. We also evaluated the bacterial flora at the skin-penetration area and its relation to the development of local and implant-related infection.
Patients and Methods
We prospectively followed 39 patients with arm and leg amputations fitted with transcutaneous osseointegrated titanium implants a mean of 56 months earlier (range, 132–133 months). There were 33 femoral, one tibial, four ulnar, four radial, and three humeral implants. Patients were selected during a 6-month period in 2005 and identically reevaluated after 3 years. Implant infection was defined as definite, probable, or possible based on clinical, radiologic, and microbiologic evidence.
Results
The frequency of implant infection was 5% at inclusion and 18% at followup. One patient with infection recovered owing to antibiotic treatment and another patient had the implant removed. Most implant infections had low infectious activity, and in five of the seven patients with infections, prosthetic use was not affected. The most common bacteria in superficial and deep cultures were Staphylococcus aureus and coagulase-negative staphylococci.
Conclusions
Despite frequent colonization around the skin-implant interface by potentially virulent bacteria such as Staphylococcus aureus and bacteria associated with biomedical device infections such as coagulase-negative staphylococci, this titanium implant system for bone-anchored prostheses caused few infections leading to disability or implant removal.
Level of Evidence
Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In high-income countries, chronic vascular disease is the leading cause of limb loss [22]. However, many previously healthy individuals undergo amputation secondary to trauma, neoplasia, infection, and arterial embolism [12]. The conventional way to suspend a prosthetic limb to the body is with a prosthetic socket [20]. Users of socket prostheses commonly report impaired quality of life [8, 13, 26, 27] and complications including dermatitis and infected sores [10, 21].
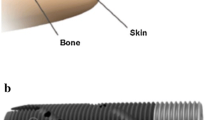
Prosthetic bone anchorage is intended to eliminate or minimize these problems. Our novel method is based on the principles of osseointegration (Fig. 1), which has been in clinical use in prosthetic teeth replacement since 1965 [4]. One report suggests a 15-year implant survival rate of approximately 90% in mandibular bone [1]. With osseointegration, direct contact between fixture and bone tissue is intended, assuring a stable, long-term attachment for the external prosthesis [5]. This technique results in easy prosthesis handling, improved limb control, and eliminates socket-impaired ROM and socket-caused skin disorders [15, 16, 30] (Fig. 2). During the years, various attempts to anchor prosthetic limbs with transcutaneous metal implants other than titanium [17, 18, 25] have failed, primarily owing to infection. Currently, our center has treated more than 100 patients with femoral titanium implants (Fig. 3), three with tibial implants, 15 with humeral implants, and 20 with ulnar and/or radial implants. Treatment failures were more common before we introduced a standardized treatment protocol in 1999 [14, 28].
Clinical observations have led us to believe that poor primary osseointegration increases the risk of subsequent infection. However, this is not substantiated. Experimental evidence supports antiinfectious properties of titanium compared with other biomaterials when implanted in bone and in soft tissues [2, 7]. Medical device infection is caused predominantly by staphylococci, with coagulase-negative staphylococci being the most common [32, 33]. However, as the definitive diagnosis of infection is sometimes a problem, culture-based algorithms have been developed to increase diagnostic sensitivity [3, 19, 23, 32, 33]. We therefore base our definitions of infection on clinical, radiographic, and bacteriologic findings. Although current titanium transcutaneous implants are intended to reduce infections compared with previously used metals and transcutaneous techniques, there are little data regarding infections with these implants. In the related but incomparable setting of dental osseointegration, infection is well characterized [31], but less has been published regarding infection in bone-anchored hearing aids [29].
Our aims were (1) to describe the frequency and (2) the bacterial flora at the skin-penetration area and its relation to the development of local and implant-related infection in this novel method. The various clinical presentations of implant infections also are described.
Patients and Methods
We prospectively studied bacterial colonization and infectious complications in 39 patients with arm and leg amputations previously treated with 45 transcutaneous osseointegrated titanium implants. All patients attending the osseointegration outpatient clinic at Sahlgrenska University Hospital, Göteborg, Sweden, for scheduled or emergency visits between January and June 2005 were included. No patients refused to participate. At least 3 months had elapsed since the second surgical procedure (abutment insertion). This cohort was followed longitudinally for an average of 3 years to identify implant infections and cross sectionally surveyed twice (at inclusion and after approximately 3 years) for bacterial presence, local infection, and antibiotic use. All skin-penetrating loci were subjected to separate clinical, bacteriologic, and radiographic assessment. There were 18 women and 21 men with a mean age of 49.3 years (range, 28–74 years). Amputations were the result of either trauma or neoplasia. Thirty-three of the implants were femoral (bilateral in one), four each were ulnar and radial (bilateral in one), three were humeral, and one was tibial. The patients had been living with the implant(s) a mean of 54 months (range, 3–132 months). Indications for these implants were severe discomfort when using conventional socket prostheses or poor stump conditions [13].
The treatment involves two separate surgical procedures. A titanium screw (fixture) is inserted into the residual bone and allowed to integrate for 6 months before the skin-penetrating extension (abutment) is inserted [28]. Based on the experience of successful skin-penetrating implants in the head and neck regions [29], the skin is attached directly to the distal end of the residual bone to reduce soft tissue mobility and risk of infection. We consider the implant osseointegrated when it is stable on clinical examination, pain free when loaded [28], and there are no signs of loosening seen on radiographs, ie, no radiolucent zone around the implant. Postoperative followup includes clinical examination (pain evaluation, implant stability, skin and soft tissue condition), a rehabilitation protocol [14], and radiographs at 6 months and 1, 2, 3, 5, 7, 10, and 15 years after surgery.
We used the following five definitions for implant infection and required all stated criteria for the given diagnosis: (1) definite implant infection: (a) clinical symptoms of implant-related infection, (b) radiographic signs consistent with periimplant bone infection, and (c) at least three of five intraoperatively obtained cultures yielding identical pathogens; (2) probable implant infection: (a) clinical symptoms of implant-related infection, (b) radiographic signs consistent with periimplant bone infection, and (c) positive relevant culture not as defined previously; (3) possible implant infection: (a) clinical symptoms of implant-related infection, (b) radiographic signs consistent with periimplant bone infection, and (c) no relevant cultures; (4) local infection in the skin penetration area: (a) local signs/symptoms of infection (inflammation with or without secretion) in the skin penetration area but no symptoms of deep infection, (b) no radiographic signs consistent with periimplant bone infection, and (c) with or without relevant cultures; (5) bacterial colonization around the skin-implant interface: (a) neither local inflammatory signs nor other clinical symptoms of infection, with or without secretion, at the skin-implant interface, (b) no radiographic signs consistent with periimplant bone infection, and (c) positive bacterial culture from the skin-implant interface. We considered radiographic evidence of osteolysis with or without periosteal sclerosis around a previously integrated implant to be consistent with implant infection. The definitions also were based on previously proposed culture diagnostics in infected hip and knee arthroplasties [19, 23].
At inclusion patients were examined clinically and asked to answer a questionnaire (Appendix 1) regarding infectious complications and antibiotic use during the 6 months preceding the visit. Without any preparation of the surrounding skin, samples were taken from the skin-implant interface with a sterile cotton swab, transported in a coal-based medium, and cultured on routine agar plates for at least 2 days. A second set of bacterial cultures was performed 3 years later and an identical questionnaire was answered. Patients who were not scheduled for a visit at this time collected bacterial samples (cotton swabs) themselves according to careful written instructions and, if necessary, with the assistance of their attending clinic. These patients were experienced in handling the skin-implant area and we anticipated no methodologic problems with the patients performing their own cultures. Cultures and questionnaires were sent to Sahlgrenska University Hospital by mail. Sampled cultures transported in a coal-based medium are expected to survive a 2-day transport by mail. Culturing the samples then was performed at our certified laboratory.
All cultures were examined by one (SK) experienced bacteriologic analyst. The number of colony forming units (CFU) was defined as +++ (> 100 CFU), ++ (10–100 CFU), or + (< 10 CFU). A routine disk method was used to determine antibiotic resistance.
The latest scheduled or symptom-prompted radiographs at inclusion and followup were checked for signs of bone infection. All patients had radiographs performed within 6 months of inclusion. Followup radiographs were obtained for 31 patients. Of the remaining eight patients, two already had osteomyelitic changes at the beginning of the study, four were excluded, and two were without infectious symptoms.
Four patients were lost to followup. In one, the implant was extracted owing to mechanical loosening in a previously radiated femur (October 2006). In another patient, chronic skin infection led to abutment removal (February 2007). Complete skin healing over the retained implant followed. Two patients (one Swedish) did not complete the followup protocol for nonmedical reasons. Their admitting medical centers were contacted and no implant infections were reported. The study was approved by the ethical committee at the University of Gothenburg.
Results
The frequencies for patients with definite/probable/possible implant infections were 5% (two of 39) at inclusion and 18% (seven of 39) at followup (Table 1). Seven patients had a history of local infection at the skin penetration area during the 6-month period before inclusion and 11 patients had local infections during the 6-month period before followup (Table 1). Four and six patients, respectively, had been treated with short-term oral antibiotics. Fourteen patients had secretion from the skin pocket. In 10 patients, the secretion was purulent.
The most common bacteria found around the skin-implant interface were Staphylococcus aureus, coagulase-negative staphylococci, and streptococci group A, B, or G. In three of seven patients with implant infections at followup, inclusion cultures from the skin-implant interface yielded the same species as the suspected infectious agent (Table 2). Staphylococcus aureus was cultured from specimens from 16 patients (17 implants), coagulase-negative staphylococci from 10, streptococcus group B, G, or nontypable from nine, beta-hemolytic streptococcus group A from one, Enterococcus sp. from three, alfa streptococci from one, and Gram-negative rods from four (Table 2) at inclusion. No Staphylococcus aureus strain was methicillin-resistant.
Followup culture specimens were obtained from 30 patients, as five patients did not send specimens as instructed. Staphylococcus aureus was still the most common finding (19) followed by coagulase-negative staphylococci (11), streptococcus group B (3), Serratia sp. (2) Enterococcus sp., alfa streptococcus, coryneforms, and Pseudomonas aeruginosa (one each) (Table 2). No Staphylococcus aureus strain was methicillin-resistant. Eight of 13 patients with recultured specimens with Staphylococcus aureus at inclusion also had Staphylococcus aureus isolated at followup.
The clinical presentation varied. Two patients had chronic skin fistulas to the implant with occasional secretion but without pain, fever, or implant loosening. Fistulas were present more than 5 years before inclusion and were not treated with antibiotics during the observation time. They were registered as implant infections at inclusion and at followup. Staphylococcus aureus and coagulase-negative staphylococci were suspected etiologic agents. Two patients had implant infections with poor primary osseointegration. One had an infected femoral implant extracted. Preoperative cultures showed coagulase-negative staphylococci, alfa streptococci, and Peptostreptococcus sp. The other patient had a humeral implant infection caused by Escherichia coli. It was verified by biopsies of the culture specimens and treated with ciprofloxacin for 6 months. One year later, there were no signs of relapse. Distal infection involving bone and soft tissue was seen in two patients with good primary osseointegration. The etiology was mixed with Staphylococcus aureus/coagulase-negative staphylococci and Staphylococcus aureus/Enterococcus faecalis being suspected. Both patients underwent surgical revision and prolonged antibiotic treatment. Acute osteomyelitis occurred in one patient who had no fever but had acute pain develop in an osseointegrated femur. Radiography revealed signs of osteomyelitis at the midfixture level. Intraoperative cultures yielded Staphylococcus aureus and coagulase-negative staphylococci (Table 3). In two of seven patients with implant infections at followup, prosthetic use was not affected at any time, three patients were affected only briefly during the time around surgical intervention, and for the patient with acute osteomyelitis, the treatment outcome is still pending.
Discussion
Transcutaneous osseointegrated titanium implants for prosthetic systems in patients with amputations provide improved performance and less socket-caused complications in selected patients [15, 16]. Our aim was to describe the frequency, clinical presentation, and bacterial occurrence in local and implant-related infections.
We acknowledge several limitations. First is the relatively short followup, although some patients had been living with the implant for more than 10 years. Most patients were young or middle-aged and probably will require use of their implants or other prostheses for numerous decades. We cannot predict the long-term durability of these implants. Second, we included some patients initially treated during a learning phase. The enrolled patients represent all phases of method development. We presume with experience the infection rates will diminish. Third, we did not review all patients. Rather, we studied a subgroup of 39 patients recruited during a 6-month period. As all patients attending (regular and emergency visits) the clinic during the inclusion period agreed to participate, we presume this cohort represents the entire population. A retrospective analysis of implant infection and risk factors in 100 patients treated with femoral osseointegrated implants and a prospective analysis of patients treated with the refined protocol are in progress.
Skin-penetrating implants were long regarded as unattainable owing to the high rates of failure caused by infection. The transcutaneous osseointegrated titanium implant challenges this claim. In our cohort, seven of 39 (18%) patients either had an implant infection or one developed within 3 years. The implant-infection/implant-year ratio (7/135) is slightly less (5%) than comparable results from Queen Mary’s Hospital in London where four of 16 patients treated with the same method had an implant-related infection develop during a cumulative 67-year period (6%) (unpublished data, Sooriakumaran S, Robinson KP, Ward DA, D′Arcy R, Chittoor SN, Written communication from 12th ISPO Congress in Vancouver, Canada, July 29, 2007). In our cohort, hematogenous and ascending bacterial spread must be suspected. Poor primary osseointegration may have facilitated an ascending infection as seen in two patients. Only one of seven patients was diagnosed and treated for an implant-related infection earlier than 31 months (mean, 34 months) after the surgical procedure. With arthroplasty, late infection (> 24 months) is considered more indicative of hematogenous seeding compared with early (< 3 months) or delayed (3–24 months) infections [33]. In our four cases with more virulent bacteria, late onset of symptoms clearly indicates that intraoperative contamination was not the cause of infection. However, we cannot know whether some of the infections were either of hematogenous or new origin, rather than as a result of residual surgical contamination.
We assessed the presence of local skin infections twice, with a greater frequency at followup (Table 2). All episodes resolved spontaneously or with oral antibiotics indicating no deeper infectious involvement. These adverse effects must be compared with those of conventional socket prostheses. For patients using conventional prostheses, socket-related problems such as ulcers and rashes on the residual limb and restricted movement have been described [8, 13]. In two studies, dermatologic problems were reported to occur among 34% to 41% of those using socket prostheses [10, 21]. Many of these are manageable. With a bone-anchored prostheses however, no socket is needed to suspend the artificial limb and consequently no patient in our study had ulcers or contact dermatitis on the residual limb.
Although various microorganisms may cause foreign body-related infection, staphylococci are the most frequently isolated pathogens [11, 32]. In our study, approximately ½ of the patients were colonized with potentially virulent Staphylococcus aureus in the skin penetration area. Despite this, only three patients had a Staphylococcus aureus implant infection. Unfortunately, we did not perform genetic typing of the bacteria. The ability to adhere to the implant material and to promote biofilm formation are important pathogenic properties for staphylococci and other bacteria [9]. The biocompatibility is greater with titanium [oxide] compared with stainless steel and cobalt-chrome alloys [2, 7]. In osseointegration, the tight junction formed between the titanium and the bone tissue might prevent adhesion, colonization, and subsequent biofilm formation. Osseointegration also can be established and maintained in aggressive inflammatory environments such as experimental arthritis in a rabbit knee model [6] and in patients with rheumatoid arthritis [24]. The osseointegration might explain why some patients with implant infections have such slow, or even no, progressive destruction of the bone tissue with subsequent loosening. It also may explain why patients with poor primary osseointegration are more susceptible to infectious complications.
We found the osseointegration method with titanium implants and skin penetration of the titanium system in patients with leg and arm amputations caused few severe infectious complications. However, infectious complications occur in approximately two-fifths of the patients during a 3-year period, mostly as local infections in the skin penetration area and more rarely as low-activity implant-associated infections. Staphylococcus aureus and coagulase-negative staphylococci were the most commonly cultured bacteria in the skin penetration area. In our opinion, the frequency and severity of infections do not appear to be obstacles for use of this method. However, future studies with longer followup will address risk factors and specific infectious complications in an attempt to reduce morbidity and increase usefulness of this novel treatment.
References
Adell R, Eriksson B, Lekholm U, Brånemark PI, Jemt T. Long-term follow-up study of osseointegrated implants in the treatment of totally edentulous jaws. Int J Oral Maxillofac Implants. 1990;5:347–359.
Arens S, Schlegel U, Printzen G, Ziegler WJ, Perren SM, Hansis M. Influence of materials for fixation implants on local infection: an experimental study of steel versus titanium DCP in rabbits. J Bone Joint Surg Br. 1996;78:647–651.
Atkins BL, Athanasou N, Deeks JJ, Crook DW, Simpson H, Peto TE, McLardy-Smith P, Berendt AR. Prospective evaluation of criteria for microbiological diagnosis of prosthetic-joint infection at revision arthroplasty: the OSIRIS Collaborative Study Group. J Clin Microbiol. 1998;36:2932–2939.
Brånemark PI, Hansson BO, Adell R, Breine U, Lindström J, Hallén O, Ohman A. Osseointegrated implants in the treatment of the edentulous jaw: experience from a 10-year period. Scand J Plast Reconstr Surg Suppl. 1977;16:1–132.
Brånemark R, Brånemark PI, Rydevik B, Myers RR. Osseointegration in skeletal reconstruction and rehabilitation: a review. J Rehabil Res Dev. 2001;38:175–181.
Brånemark R, Thomsen P. Biomechanical and morphological studies on osseointegration in immunological arthritis in rabbits. Scand J Plast Reconstr Surg Hand Surg. 1997;31:185–195.
Cordero J, Munuera L, Folgueira MD. Influence of metal implants on infection: an experimental study in rabbits. J Bone Joint Surg Br. 1994;76:717–720.
Demet K, Martinet N, Guillemin F, Paysant J, André JM. Health related quality of life and related factors in 539 persons with amputation of upper and lower limb. Disabil Rehabil. 2003;25:480–486.
Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–193.
Dudek NL, Marks MB, Marshall SC, Chardon JP. Dermatologic conditions associated with use of a lower-extremity prosthesis. Arch Phys Med Rehabil. 2005;86:659–663.
Geipel U, Herrmann M. [The infected implant. Part 1: bacteriology] [in German]. (Erratum in: Orthopade. 2005;34:119. Dosage error in article text.) Orthopade. 2004;33:1411–1426; 1427–1428.
Global Lower Extremity Amputation Study Group. Epidemiology of lower extremity amputation in centres in Europe, North America and East Asia: the Global Lower Extremity Amputation Study Group. Br J Surg. 2000;87:328–337.
Hagberg K, Brånemark R. Consequences of non-vascular trans-femoral amputation: a survey of quality of life, prosthetic use and problems. Prosthet Orthot Int. 2001;25:186–194.
Hagberg K, Brånemark R. One hundred patients treated with osseointegrated transfemoral amputation prostheses: rehabilitation perspective. J Rehabil Res Dev. 2009;46:331–344.
Hagberg K, Brånemark R, Gunterberg B, Rydevik B. Osseointegrated trans-femoral amputation prostheses: prospective results of general and condition-specific quality of life in 18 patients at 2-year follow-up. Prosthet Orthot Int. 2008;32:29–41.
Hagberg K, Häggström E, Uden M, Brånemark R. Socket versus bone-anchored trans-femoral prostheses: hip range of motion and sitting comfort. Prosthet Orthot Int. 2005;29:153–163.
Hall CW. A future prosthetic limb device. J Rehabil Res Dev. 1985;22:99–102.
Hall CW, Cox PA, Mallow WA. Skeletal extension development: criteria for future designs. Bull Prosthet Res. 1976;Spring (10–25):69–96.
Kamme C, Lindberg L. Aerobic and anaerobic bacteria in deep infections after total hip arthroplasty: differential diagnosis between infectious and non-infectious loosening. Clin Orthop Relat Res. 1981;154:201–207.
Kapp S. Suspension systems for prostheses. Clin Orthop Relat Res. 1999;361:55–62.
Lyon CC, Kulkarni J, Zimerson E, Van Ross E, Beck MH. Skin disorders in amputees. J Am Acad Dermatol. 2000;42:501–507.
Marks LJ, Michael JW. Science, medicine, and the future: artificial limbs. BMJ. 2001;323:732–735.
Mikkelsen DB, Pedersen C, Højbjerg T, Schønheyder HC. Culture of multiple peroperative biopsies and diagnosis of infected knee arthroplasties. APMIS. 2006;114:449–452.
Möller K, Geijer M, Sollerman C, Lundborg G. Radiographic evaluation of osseointegration and loosening of titanium implants in the MCP and PIP joints. J Hand Surg Am. 2004;29:32–38.
Mooney V, Schwartz SA, Roth AM, Gorniowsky MJ. Percutaneous implant devices. Ann Biomed Eng. 1977;5:34–46.
Pezzin LE, Dillingham TR, MacKenzie EJ. Rehabilitation and the long-term outcomes of persons with trauma-related amputations. Arch Phys Med Rehabil. 2000;81:292–300.
Pezzin LE, Dillingham TR, Mackenzie EJ, Ephraim P, Rossbach P. Use and satisfaction with prosthetic limb devices and related services. Arch Phys Med Rehabil. 2004;85:723–729.
Robinson KP, Brånemark R, Ward D. Future developments: osseointegration in transfemoral amputees. In: Smith DG, Michael JW, Bowker JH, eds. Atlas of Amputations and Limb Deficiencies: Surgical, Prosthetic and Rehabilitation principles. Ed 3. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2004:673–681.
Snik AF, Mylanus EA, Proops DW, Wolfaardt JF, Hodgetts WE, Somers T, Niparko JK, Wazen JJ, Sterkers O, Cremers CW, Tjellström A. Consensus statements on the BAHA system: where do we stand at present? Ann Otol Rhinol Laryngol Suppl. 2005;195:2–12.
Sullivan J, Uden M, Robinson KP, Sooriakumaran S. Rehabilitation of the trans-femoral amputee with an osseointegrated prosthesis: the United Kingdom experience. Prosthet Orthot Int. 2003;27:114–1120.
Tabanella G, Nowzari H, Slots J. Clinical and microbiological determinants of ailing dental implants. Clin Implant Dent Relat Res. 2009;11:24–36.
von Eiff C, Jansen B, Kohnen W, Becker K. Infections associated with medical devices: pathogenesis, management and prophylaxis. Drugs. 2005;65:179–214. Review.
Zimmerli W, Ochsner PE. Management of infection associated with prosthetic joints. Infection. 2003;31:99–108.
Acknowledgments
We thank Samir Kawash at the Clinical Bacteriological Laboratory at Sahlgrens University Hospital for invaluable help.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
One or more of the authors (JT, LH) have received funding from the LUA project (ALFGBG-11128), University of Gothenburg, Göteborg, Sweden.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
Appendix 1
Appendix 1

Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Tillander, J., Hagberg, K., Hagberg, L. et al. Osseointegrated Titanium Implants for Limb Prostheses Attachments: Infectious Complications. Clin Orthop Relat Res 468, 2781–2788 (2010). https://doi.org/10.1007/s11999-010-1370-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-010-1370-0