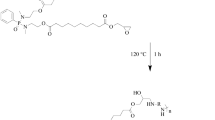
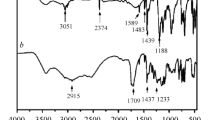
Abstract
Materials imparting flame retardancy play a pivotal role in the development of advanced materials due to their ability to reduce the annihilating effect of fire on man and environment. Realizing the commercial significance of epoxy resins, an attempt has been made to enhance their flame retardancy even under demanding conditions. Among the various flame-retardant materials, phosphorus-containing compounds are gaining interest both in academic and industrial areas due to their simple mechanism of action which involves char formation, a barrier between the substrate and the fire which protects the substrate from being burnt. In the present research work, unconventional starting material—diethanolamine—was used to synthesize phosphorus-based flame-retardant precursors. Intermediates formed in first step were reacted with epichlorohydrin to form epoxy resins. Structure of the compounds was confirmed by Fourier transform infrared spectroscopy, nuclear magnetic resonance spectroscopy, hydroxyl, and epoxy values. Resins were cured with commercial polyamide, and properties were compared with commercial epoxy resin. Coating films were characterized by various physical, mechanical, and thermal properties like impact resistance, pencil hardness, gloss, solvent resistance, thermogravimetric analysis (TGA), and differential scanning calorimetry (DSC). The TGA and DSC results showed that the char yield value has increased from 3.36 to 8.53%, and the glass transition temperature (Tg) has increased from 72.86 to 82.05°C while the initial degradation temperature decreased. Coatings showed excellent flame retardancy with maximum Limiting Oxygen Index value of 35 and a self-extinguishing behavior as confirmed by the UL-94 test.











Similar content being viewed by others
References
Dewprashad, B, “Fundamentals of Epoxy Formulation.” J. Chem. Educ., 71 290–294 (1946)
Ashcroft, WR, “Curing Agents for Epoxy Resins.” In: Ellis, B (ed.) Chemistry and Technology of Epoxy Resins, pp. 37–71. Springer, Dordrecht (1993)
Gibson, G, “Epoxy Resins.” In: Brydson, JA (ed.) Brydson’s Plastics Materials, pp. 773–797. Elsevier, Amsterdam (2017) https://doi.org/10.1016/b978-0-323-35824-8.00027-x
Ramos, D, Helson, M, Soares, V, Nascimento, R, “Modification of Epoxy Resin: A Comparison of Different Types of Elastomer.” Polym. Test., 24 387–394 (2005). https://doi.org/10.1016/j.polymertesting.2004.09.010
Chikhi, N, Fellahi, S, Bakar, M, “Modification of Epoxy Resin Using Reactive Liquid (ATBN) Rubber.” Eur. Polym. J., 38 251–264 (2002). https://doi.org/10.1016/S0014-3057(01)00194-X
Cheng, X, Chen, Y, Du, Z, Zhu, P, Wu, D, “Effect of the Structure of Curing Agents Modified by Epoxidized Oleic Esters on the Toughness of Cured Epoxy Resins.” J. Appl. Polym. Sci., 119 3504–3510 (2010). https://doi.org/10.1002/app.32998
Ross, H, Coscia, T, “Some Reactions of Epichlorohydrin with Amines.” J. Org. Chem., 29 824–826 (1964). https://doi.org/10.1021/jo01027a012
Cross, RP, Fugassi, P, Branch, K, Calvin, M, “The Reaction of Epichlorohydrin with Secondary Amines.” J. Am. Chem. Soc. , 80 (5) 1257–1259 (1964). https://doi.org/10.1021/ja01538a057
Christopher, M, Dobinson, B, Rolfe, M, Michael, R, “Glycidyl Ether from Alcohol and Epichlorohydrin.” US Patent 5,420,312, 1995
Bukowska, A, Bukowski, W, “Reactivity of Some Carboxylic Acids in Reactions with Some Epoxides in the Presence Chromium (III) Ethanoate.” Org. Process Res. Dev., 6 (3) 234–237 (2002). https://doi.org/10.1021/op010112q
Bukowski, W, “The Solvent Effects in the Reactions of Carboxylic Acids with Oxiranes 1 Kinetics of the Reaction of Acetic Acid with Epichlorohydrin in Butan-1-ol.” Int. J. Chem. Kinet., 32 (6) 378–387 (2000). https://doi.org/10.1002/(sici)1097-4601(2000)32:6<378::aid-kin4>3.0.co;2-3
Saurabh, T, Patnaik, M, Bhagt, SL, Renge, VC, “Epoxidation of Vegetable Oils: A Review.” Int. J. Adv. Eng. Technol., 2 (4) 491–501 (2011)
Jian, X, Hay, AS, “Epoxidation of Unsaturated Polymers with Hydrogen Peroxide.” J. Polym. Sci. C, 28 285–288 (1990). https://doi.org/10.1002/pol.1990.140280903
Caillol, S, David, G, Boutevin, B, Pascault, JP, “Biobased Thermosetting Epoxy: Present and Future.” Chem. Rev., 114 1082–1115 (2014). https://doi.org/10.1021/cr3001274
Baiker, JJ, Cofer, KB, Leonard, H, Sheets, DM, “Synthesis of glycidyl ethers of polyhydric phenols.” US Patent 3,121,727 (1964)
Saxena, NK, Gupta, DR, “Development and Evaluation of Fire Retardant Coatings.” Fire Technol., 26 (4) 329–341 (1990). https://doi.org/10.1007/BF01293077
Weil, ED, “Flame-Retardant Coatings—A State-of-the-Art Review.” J. Fire Sci. (2011). https://doi.org/10.1177/0734904110395469
Weil, ED, Levchik, SV, “Flame Retardants in Commercial Use or Development for Textiles.” J. Fire Sci., 26 243–281 (2011). https://doi.org/10.1177/0734904108089485
Szolnoki, B, Toldy, A, Konrád, P, Szebényi, G, Marosi, G, “Comparison of Additive and Reactive Phosphorus-Based Flame Retardants in Epoxy Resins.” Period. Polytech. Chem. Eng., 2 85–91 (2013). https://doi.org/10.3311/ppch.2175
Reistad, T, Mariussen, E, Fonnum, F, “The Effect of a Brominated Flame Retardant, Tetrabromobisphenol-A, on Free Radical Formation in Human Neutrophil Granulocytes: The Involvement of the MAP Kinase Pathway and Protein Kinase C.” Toxicol. Sci., 83 89–100 (2005). https://doi.org/10.1093/toxsci/kfh298
Tirri, T, Aubert, M, Pawelec, W, Holappa, A, Wilén, C, “Structure–Property Studies on a New Family of Halogen Free Flame Retardants Based on Sulfenamide and Related Structures.” Polymers, 8 (10) 360 (2016). https://doi.org/10.3390/polym8100360
Schartel, B, “Phosphorus-Based Flame Retardancy Mechanisms—Old Hat.” Materials, 3 4710–4745 (2010). https://doi.org/10.3390/ma3104710
Jeng, R, Shau, S, Lin, J, Su, W, Chiu, Y, “Flame Retardant Epoxy Polymers Based on All Phosphorus-Containing Components.” Eur. Polym. J., 38 683–693 (2002). https://doi.org/10.1016/S0014-3057(01)00246-4
Gu, J-W, Zhang, G-C, Dong, S-L, Zhang, Q-U, Kong, J, “Study on Preparation and Fire-Retardant Mechanism Analysis of Intumescent Flame-Retardant Coatings.” Surf. Coat. Technol., 201 7835–7841 (2007). https://doi.org/10.1016/j.surfcoat.2007.03.020
Yanga, X, Guoa, Y, Luob, X, Zhenga, N, Maa, T, Tana, J, Lia, C, Zhanga, Q, Gu, J, “Self-Healing, Recoverable Epoxy Elastomers and Their Composites with Desirable Thermal Conductivities by Incorporating BN Fillers Via In-Situ Polymerization.” Compos. Sci. Technol., 164 59–64 (2018). https://doi.org/10.1016/j.compscitech.2018.05.038
Nygaard, D, Diguilio, M, Rock, R, “Production of Diethanolamine.” US Patent 6,063,965 (2000)
Frauenkron, M, Melder, J, Ruider, G, Rossbacher, R, Hoke, H, “Ethanolamines and Propanolamines.” In: Elvers, B (ed.) Ullmann’s Encyclopedia of Industrial Chemistry, vol. 13, p. 405. https://doi.org/10.1002/14356007.a10 (2012)
Patil, DM, Phalak, GA, Mhaske, ST, “Synthesis of Bio-Based Epoxy Resin from Gallic Acid with Various Epoxy Equivalent Weights and Its Effects on Coating Properties.” J. Coat. Technol. Res., 14 (2) 355–365 (2017). https://doi.org/10.1007/s11998-016-9853-x
Chen, X, Jiao, C, “Thermal Degradation Characteristics of a Novel Flame Retardant Coating Using TG-IR Technique.” Polym. Degrad. Stab., 93 2222–2225 (2008). https://doi.org/10.1016/j.polymdegradstab.2008.09.005
Vlad-bubulac, T, Hamciuc, C, Petreus, O, “Synthesis and Properties of Some Phosphorus-Containing Polyesters.” High Perform. Polym., 18 255–264 (2005). https://doi.org/10.1177/0954008306059504
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mestry, S., Mhaske, S.T. Synthesis of epoxy resins using phosphorus-based precursors for flame-retardant coating. J Coat Technol Res 16, 807–818 (2019). https://doi.org/10.1007/s11998-018-00157-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-018-00157-3