Abstract
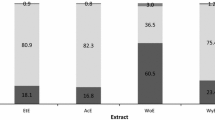
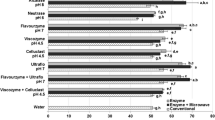
In this study, antioxidant activity of absolute ethanol, 50 % ethanol and water extracts of two species of seaweeds namely, Fucus serratus and Polysiphonia fucoides were evaluated for their ability to retard lipid and protein oxidation in minced mackerel. Mackerel mince added with 0.5 g/kg of extracts was prepared. For comparison, BHT at 0.2 g/kg and a control with no added extracts were also prepared. The samples were stored at 5 °C for 8 days, and sampling was done at time 0, 1, 2, 4, 5 and 8 days. The 50 % ethanolic extracts of P. fucoides were found to be very effective in retarding lipid and protein oxidation, as it resulted in low levels of peroxide value, volatiles and carbonyl compounds and protected against the loss of α-tocopherol and tryptophan residues. In spite of the higher phenolic content, the absolute ethanol extracts of both species showed a pro-oxidative tendency in minced mackerel. Water extract with lowest phenolic content showed no antioxidant effect in minced mackerel. In conclusion, the 50 % ethanolic extracts of P. fucoides can be a potential source of natural antioxidants, as these extracts have antioxidant activities similar to synthetic antioxidants such as BHT. However, the extent of protection offered by these extracts against protein oxidation was not clear and further studies are needed to understand the nature of the interaction between proteins and these extracts.



Similar content being viewed by others
References
AOAC. (1995). Official method of analysis (16th ed.). Arlington: AOAC.
AOCS. (1992). Official method Ce 8-89. Determination of tocopherols and tocotrienols in vegetable oils and fats by HPLC. Champaign: AOCS.
AOCS. (1998). Official methods, and recommended practices (5th ed.). Champaign: AOCS.
Bader, N., & Grune, T. (2006). Protein oxidation and proteolysis. Biological Chemistry, 387, 1351–1355.
Baron, C. P., Kjærsgård, I. V. H., Jessen, F., & Jacobsen, C. (2007). Protein and lipid oxidation during frozen storage of rainbow trout (Oncorhynchus mykiss). Journal of Agricultural and Food Chemistry, 55, 8118–8125.
Bligh, E. G., & Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37, 911–917.
Boyd, L. C., Green, D. P., Giesbrecht, F. B., & King, M. F. (1993). Inhibition of oxidative rancidity in frozen cooked fish flakes by tert-butylhydroquinone and rosemary extract. Journal of Science of Food and Agriculture, 61, 87–93.
Chandini, S. K., Ganesan, P., & Bhaskar, N. (2008). In vitro antioxidant activities of three selected brown seaweeds of India. Food Chemistry, 107(2), 707–713.
Chojnacka, K., Saeid, A., Witkowska, Z., & Tuhy, Ł. (2012). Biologically active compounds in seaweed extracts—the prospects for the application. The Open Conference Proceedings Journal, 3, 20–28.
Connor, W. E. (2000). Importance of n-3 fatty acids in health and disease. American Journal of Clinical Nutrition, 71, 171s–175s.
Dalle-Donne, I., Rossi, R., Giustarini, D., Gagliano, N., Lusini, L., Milzani, A., et al. (2001). Actin carbonylation: from a simple marker of protein oxidation to relevant signs of severe functional impairment. Free Radical Biology and Medicine, 2001(31), 1075–1083.
Dalle-Donnea, I., Rossib, R., Giustarinib, D., Milzania, A., & Colombo, R. (2003). Protein carbonyl groups as biomarkers of oxidative stress. Review. Clinica Chimica Acta, 329, 23–38.
Davies, K. J. A., Delsignore, M. E., & Lin, S. W. (1987). Protein damage and degradation by oxygen radicals. I. Modification of amino acids. Journal of Biological Chemistry, 262, 9902–9907.
El-Agamey, A., Lowe, G. M., Mc Garvey, D. J., Mortensen, A., Phillip, D. M., Truscott, T. G., & Young, A. J. (2004). Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Archives of Biochemistry and Biophysics, 430, 37–48.
Ellman, G. L. (1959). Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics, 82, 70–77.
Farvin, K. H. S., & Jacobsen, C. (2013). Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chemistry, 138(2,3), 1670–1681.
Farvin, K. H. S., & Jacobsen, C. (2015). Antioxidant activity of seaweed extracts: in vitro assays, evaluation in 5 % fish oil-in-water emulsions and characterization. Journal of the American Oil Chemists’ Society, 92(4), 571–587.
Farvin, K. H. S., Grejsen, H. D., & Jacobsen, C. (2012). Potato peel extract as a natural antioxidant in chilled storage of minced horse mackerel (Trachurus trachurus): effect on lipid and protein oxidation. Food Chemistry, 131, 843–851.
Frankel, E. N. (2005). In E. N. Frankel (Ed.), Lipid oxidation (2nd ed.). Bridgwater: The Oily Press, P.J. Barnes Associates.
Fujimoto, K., Ohmura, H., & Kaneda, T. (1985). Screening for antioxygenic compounds in marine algae and bromophenols as effective principles in a red algae Polysiphonia ulceolate. Bulletin of the Japanese Society of Scientific Fisheries, 51, 1139–1143.
Gardner, H. W. (1979). Lipid hydroperoxides reactivity with proteins and amino acids: a review. Journal of Agricultural and Food Chemistry, 43, 651–656.
Gelamo, E. L., Silva, C. H. T. P., Imasato, H., & Tabak, M. (2002). Interaction of bovine (BSA) and human (HSA) serum albumins with ionic surfactants: spectroscopy and modeling. Biochimica et Biophysica Acta, 1594, 84–99.
Gupta, S., & Abu-Ghannam, N. (2011). Bioactive potential and possible health effects of edible brown seaweeds. Trends in Food Science & Technology, 22, 315–326.
Hayakawa, S., & Nakai, S. (1985). Contribution of hydrophobicity, net charge and sulfhydryl groups to thermal properties of ovalbumin. Canadian Institute of Food Science Technology Journal, 18, 290–295.
Hermund, D. B., Iltas¸, B. Y., Honold, P., Jónsdóttir, R., Kristinsson, H. G., & Jacobsen, C. (2015). Characterisation and antioxidant evaluation of Icelandic F. vesiculosus extracts in vitro and in fish-oil-enriched milk and mayonnaise. Journal of Functional Foods. doi:10.1016/ j.jff.2015.02.020.
Hidalgo, F. J., & Kinsella, J. E. (1989). Changes induced in â-lactoglobulin B following interactions with linoleic acid 13-hydroperoxide. Journal of Agricultural and Food Chemistry, 37, 860–866.
International IDF Standards. (1991). Section 74A: 1991. International Dairy Federation, IDF-square Vergot 41. Belgium: Brussels.
Kristinsson, H. G., Theodore, A. E., Demir, N., & Ingadottir, B. (2005). A comparative study between acid- and alkali- aided processing and surimi processing for the recovery of proteins from channel catfish muscle. Journal of Food Science, 70, C298–C306.
Ladikos, D., & Lougovois, V. (1990). Lipid oxidation in muscle foods: a review. Food Chemistry, 35, 295–314.
Lanier, T. C. (2000). Surimi gelation chemistry. In J. W. Park (Ed.), Surimi and surimi seafood (pp. 237–265). New York: Marcel Dekker.
Levine, R. L., Williams, J. A., Stadtman, E. R., & Shacter, E. (1994). Carbonyl assays for determination of oxidatively modified proteins. Methods in Enzymology, 233, 346–357.
Linderschmidt, R., Trylka, A., Goad, M., & Witschi, H. (1986). The effects of dietary butylated hydroxytoluene on liver and colon tumour development in mice. Toxicology, 38, 151–160.
Little, C., & O’Brien, P. J. (1967). Products of oxidation of a protein thiol group after reaction with various oxidizing agents. Archives of Biochemistry and Biophysics, 122, 406–410.
Medina, I., González, M. J., Iglesias, J., & Hedges, N. D. (2009). Effect of hydroxy cinnamic acids on lipid oxidation and protein changes as well as water holding capacity in frozen minced horse mackerel white muscle. Food Chemistry, 114, 881–888.
Moroney, N. C., O’Grady, M. N., O’Doherty, J. V., & Kerry, J. P. (2013). Effect of a brown seaweed (Laminaria digitata) extract containing laminarin and fucoidan on the quality and shelf-life of fresh and cooked minced pork patties. Meat Science, 94, 304–311.
O’Sullivan, A., O’Callaghan, Y., O’Grady, M., Queguineur, B., Hanniffy, D., Troy, D., Kerry, J., & O’Brien, N. (2011). In vitro and cellular antioxidant activities of seaweed extracts prepared from five brown seaweeds harvested in spring from the west coast of Ireland. Food Chemistry, 126(3), 1064–1070.
Ortiz, J., Vivanco, J. P., & Aubourg, S. P. (2014). Lipid and sensory quality of canned Atlantic salmon (Salmo salar): effect of the use of different seaweed extracts as covering liquids. European Journal of Lipid Science and Technology, 116(5), 596–605.
Ragan, M. A., & Glombitza, K. W. (1986). Physodes and the phenolic compounds of brown algae 4. Oligomeric polyphloroglucinols from Fucus vesiculosus—photoplate mass-spectrometric investigation. Phytochemistry, 21, 2709–2711.
Rawel, H. M., Czajka, D., Rohn, S., & Kroll, J. (2002a). Interactions of different phenolic acids and flavonoids with soy proteins. International Journal of Biological Macromolecules, 30, 137–150.
Rawel, H. M., Rohn, S., Kruse, H.-P., & Kroll, J. (2002b). Structural changes induced in bovine serum albumin by covalent attachment of chlorogenic acid. Food Chemistry, 78, 443–455.
Ribeiro, I.S., Shirahigue, L.D., Sucasas, L.F.A., Anbe, L., Cruz, P.G., Gallo, C.R., et al. (2014). Shelf life and quality study of minced tilapia with Nori and Hijiki seaweeds as natural additives. Scientific World Journal, Article ID 485287, 7 pages. doi:10.1155/2014/485287.
Shahidi, F., & Miraliakbari, H. (2004). Omega-3 (n-3) fatty acids in health and disease: part 1—cardiovascular disease and cancer. Journal of Medicinal Food, 7(4), 387–401.
Shi, H., Noguchi, N., & Nike, T. (2000). Natural antioxidants. In J. Pokorny, N. Yanishlieva, & M. Gordon (Eds.), Antioxidants in food (pp. 147–155). Boca Raton: CRC Press.
Suryaprakash, P., Kumar, R. P., & Prakash, V. (2000). Thermodynamics of interaction of caffeic acid and quinic acid with multi subunit proteins. International Journal of Biological Macromolecules, 27, 219–228.
Tesoriere, L., Butera, D., Gentile, C., & Livrea, M. A. (2007). Bioactive components of caper (Capparis spinosa L.) from Sicily and antioxidant effects in a red meat simulated gastric digestion. Journal of Agricultural and Food Chemistry, 55, 8465–8471.
Wang, T., Jónsdóttir, R., & Ólafsdóttir, G. (2009). Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chemistry, 116, 240–248.
Yan, X., Nagata, T., & Fan, X. (1998). Antioxidative activities in some common seaweeds. Plant Foods for Human Nutrition, 52, 253–262.
Yangthong, M., Hutadilok-Towatana, N., & Phromkunthong, W. (2009). Antioxidant activities of four edible seaweeds from the southern coast of Thailand. Plant Foods for Human Nutrition, 64(3), 218–223.
Zamora, R., Alaiz, M., & Hidalgo, F. J. (1999). Modification of histidine residues by 4,5-epoxy-3-alkenals. Chemical Research in Toxicology, 12, 654–660.
Acknowledgments
This work was financially supported by the Danish Research Council for Technology and Production. The help provided by technician Inge Holmberg for the HPLC analysis of phenolic compounds and Susan Løvstad Holdt for collecting seaweeds is greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Babakhani, A., Farvin, K.H.S. & Jacobsen, C. Antioxidative Effect of Seaweed Extracts in Chilled Storage of Minced Atlantic Mackerel (Scomber scombrus): Effect on Lipid and Protein Oxidation. Food Bioprocess Technol 9, 352–364 (2016). https://doi.org/10.1007/s11947-015-1630-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-015-1630-9