Abstract
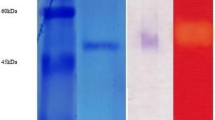
The lignocellulosic coffee by-products such as coffee pulp, coffee cherry husk, silver skin, and spent coffee were evaluated for their efficacy as a sole carbon sources for the production of xylanase in solid-state fermentation using Penicillium sp. CFR 303. Among the residues, coffee cherry husk was observed to produce maximum xylanase activity of 9,475 U/g. The process parameters such as moisture (50%), pH (5.0), temperature (30 °C), particle size (1.5 mm), inoculum size (20%), fermentation time (5 days), carbon source (xylose), and nitrogen source (peptone) were optimized and the enzyme activity was in the range of 19,560–20,388 U/g. The enzyme production was further improved to 23,494 U/g with steam as a pre-treatment. The extracellular xylanase from the fungal source was purified to homogeneity from culture supernatant by ammonium sulfate fractionation, DE32-cellulose with a recovery yield of 25.5%. It appeared as a single band on SDS-PAGE gel with a molecular mass of approximately 27 kDa. It had optimum parameters of 50 °C temperature, pH 5.0, K m 5.6 mg/mL, and V max 925 μmol mg−1 min−1 with brichwood xylan as a substrate. The crude enzyme hydrolysed lignocellulosic substrate as well as industrial pulp. Production of xylanase utilizing coffee by-products constitutes a renewable resource and is reported for the first time.






Similar content being viewed by others
References
Aguilar, G., Morton-Guyot, J., Trejo Aguilar, B., & Guyot, J. (2000). Purification and characterization of extracellular amylase produced by Lactobacillus manihotivorans LMG 18010 T, an amlylolytic lactic acid bacterium. Enzyme Microbial Technology, 27, 406–413.
Bailey, M. J., Biely, P., & Poutanen, K. (1992). Interlaboratory testing of methods for assay of xyalanase activity. Journal of Biotechnology, 23, 257–270.
Bajpai, P. (1997). Microbial xylanolytic enzyme system: Properties and applications. Advances in Applied Microbiology, 43, 141–194.
Bajpai, P. (1999). Applications of enzymes in the pulp and paper industry. Biotechnology Progress, 15(2), 147–157.
Balkan, B., & Ertan, F. (2005). Production and properties of α-amylase from Pencillium chrysogenum and its applications in starch hydrolysis. Preparative Biochemistry and Biotechnology, 35, 169–178.
Balkan, B., & Ertan, F. (2007). Production of α-amylase from Penicillium chrysogenum under solid-state fermentation by using some agricultural by-products. Food Technology and Biotechnology, 45, 439–442.
Baysal, Z., Uyar, F., & Aytekin, C. (2003). Solid-state fermentation for production of α-amylase by a thermotolerant Bacillus subtilis from hot-spring water. Process Biochemistry, 38, 1665–1668.
Beg, Q. K., Kapoor, M., Mahajan, L., & Hoondal, G. S. (2001). Microbial xylanases and their industrial applications: A review. Applied Microbiology and Biotechnology, 56, 326–338.
Christov, L. P., Szakacs, G., & Balaksrishnan, H. (1999). Production, partial characterization and use of fungal cellulose-free xylanases in pulp bleaching. Process Biochemistry, 34, 511–517.
De Souza-Querido, A. L., Cavalcante-Coelho, J. L., Fernandes de Araujo, E., & Chaves-Alves, V. M. (2006). Partial purification and characterization of xylanase produced by Penicillium expansum. Brazilian Archives of Biology and Technology, 49, 475–480.
Farga, L. P., Macedo, G. A., & Carvalho, P. O. (2009). Production of cutinase by Fusarium oxysporum on Brazilian agricultural by-products and its Enantioselective properties. Food and Bioprocess Technology, doi:10.1007/s11947-009-0261-4.
Febe, F., Sabu, A., Madhavan-Nampoothiri, K., Szakacs, G., & Pandey, A. (2002). Synthesis of α-amylase by Aspergillus oryzae in solid-state fermentation. Journal of Basic Microbiology, 42, 320–326.
Gaspar, A., Cosson, T., Roques, C., & Thonart, P. H. (1997). Study on the production of a xylanolytic complex from Penicillium canescens 10-10c. Applied Biochemistry and Biotechnology, 67, 45–58.
Heck, J. X., De Barros-Soares, L. H., Hertz, P. F., & Záchia-Ayub, M. A. (2006). Purification and properties of a xylanase produced by Bacillus circulans BL53 on solid-state cultivation. Biochemical Engineering Journal, 32, 179–184.
Jecu, L. (2000). Solid state fermentation of agricultural; wastes for endoglucanase production. Industrial Crops and Products, 11, 1–5.
Kashyap, P., Sabu, A., Pandey, A., Szakacs, G., & Soccol, C. R. (2002). Extra-cellular L-glutaminase production by Zygosaccharomyces rouxii under solid-state fermentation. Process Biochemistry, 38, 307–312.
Krishna, C., & Chandrasekaran, M. (1996). Banana waste as substrate for α-amylase production by Bacillus subtilis (CBTK 106) under solid-state fermentation. Applied Microbiology and Biotechnology, 46, 106–111.
Kuhad, R., Manchanda, M., & Singh, A. (1998). Optimization of Xylanase production by a hyperxylanolytic mutant strain of Fusarium oxysporum. Process Biochemistry, 33, 641–647.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685.
Lowry, O. H., Rosenbrough, N. J., Lewis-Farr, A., & Randall, R. J. (1951). Protein measurement with the Folin-phenol reagent. Journal of Biological Chemistry, 193, 265–275.
Mamo, G., Hatti-Kaul, R., & Mattiasson, B. (2006). A thermostable alkaline active endo-ß-1, 4-Xylanase from Bacillus halodurans S7: Purification and characterization. Enzyme Microbial Technology, 39, 1492–1498.
Meenakshi, G., Kalra, K. L., Sareen, V. K., & Soni, G. (2008). Xylanase production with xylan rich lignocellulosic wastes by a local soil isolate of Trichoderma viride. Brazilian Journal of Microbiology, 39, 535–541.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugars. Analytical Chemistry, 31, 426–428.
Murthy, P. S., & Manonmani, H. K. (2008). Bioconversion of coffee industry wastes with white rot fungi Pleurotus florida. Research Journal of Environmental Science, 2, 145–150.
Murthy, P. S., Madhava Naidu, M., & Srinivas, P. (2009). Production of α-amylase under solid-state fermentation utilizing coffee waste. Journal of Chemical Technology & Biotechnology, 84, 1246–1249.
Oberoi, H. S., Chavan, Y., Bansal, S., & Dhillon, G. S. (2008). Production of cellulases through solid state fermentation using kinnow pulp as a major substrate. Food and Bioprocess Technology, doi:10.1007/s11947-008-0092-8.
Palmer, T. (2001). Enzymes: Biochemistry, biotechnology and clinical chemistry (pp. 191–222). Chichester: Horwood Publication.
Puls, J. C., & Schuseil, J. (1993). Chemistry of hemicelluloses: Relation between hemicellulose structure and enzymes required for hydrolysis. In M. P. Coughlan & G. P. Hazlewood (Eds.), Hemicelluloses and hemicellulases (pp. 1–27). London: Portland.
Soloarzano- Lemos, J. L., & Pereira Junior, N. (2002). Influence of some sugars on xylanase production by Aspergillus awamori in solid-state fermentation. Brazilian Archives of Biology and Technology, 45, 431–437.
Souza, C. G. M., Simao, R. C. G., & Peralta, R. M. (1998). Purification and characterization of alkali tolerant xylanases from Aspergillus tamarii. Revista de Microbiologia, 29, 93–98.
Zhiwei, L. V., Jinshui, Y., & Hongli, Y. (2008). Production, purification and characterization of an alkaliphilic endo-ß-1, 4-Xylanase from a microbial community EMSD5. Enzyme and Microbial Technology, 43, 343–348.
Acknowledgments
We thank Dr. V. Prakash, Director, CFTRI, Mysore for constant encouragement. Thanks are also due to Dr. P. Srinivas, Head, Department of Plantation Products Spices and Flavour Technology, CFTRI for his support. The finical help from CSIR, New Delhi, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murthy, P.S., Naidu, M.M. Production and Application of Xylanase from Penicillium sp. Utilizing Coffee By-products. Food Bioprocess Technol 5, 657–664 (2012). https://doi.org/10.1007/s11947-010-0331-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-010-0331-7