Abstract
Consumers demand high-quality processed foods with minimal changes in nutritional and sensory properties. Nonthermal methods are considered to keep food quality attributes better than traditional thermal processing. Pulsed light (PL) is an emerging nonthermal technology for decontamination of food surfaces and food packages, consisting of short time high-peak pulses of broad spectrum white light. It is considered an alternative to continuous ultraviolet light treatments for solid and liquid foods. This paper provides a general review of the principles, mechanisms of microbial inactivation, and applications of PL treatments on foods. Critical process parameters that are needed to be optimized for a better efficiency of PL treatments are also discussed. PL has considerable potential to be implemented in the food industry. However, technological problems need to be solved in order to avoid food overheating as well as to achieve better penetration and treatment homogeneity. In addition, a more extensive research is needed to understand how PL affects quality food attributes.
Similar content being viewed by others
Introduction
Nonthermal technologies are being applied in food processing as a viable alternative to thermal processing (Guerrero-Beltrán and Barbosa-Cánovas 2004). Traditionally, most foods are thermally processed by subjecting them to temperatures between 60 °C for a few minutes and 100 °C for a few seconds. During this period, a large amount of energy is transferred to the food, which may trigger reactions that lead to undesirable changes or by-products formation. During nonthermal processing, food temperature is held below that achieved in thermal treatments. Thus, vitamins, essential nutrients, and flavors are expected to undergo minimal or no changes.
Pulsed light (PL) is used for the rapid inactivation of microorganisms on food surfaces, equipment, and food packaging materials. The terms, high intensity broad spectrum pulsed light (Roberts and Hope 2003) and pulsed white light (Marquenie et al. 2003a, b), are synonymous with PL (Rowan et al. 1999).
The use of inert-gas flash lamps generating intense and short pulses of ultraviolet (UV) light for microbial inactivation started during the late 1970s in Japan. In 1988, extensive experimentation carried out by PurePulse Technologies Inc. provided a pulsed light process called PureBright® to sterilize pharmaceuticals, medical devices, packaging, and water. The efficacy of the process was tested against a broad range of microorganisms, including bacteria (vegetative cells and spores), fungi, viruses, and protozoa. However, the technology was adopted by the food industry only in 1996, when the Food and Drug Administration approved the use of PL technology for production, processing, and handling of foods.
Description of PL
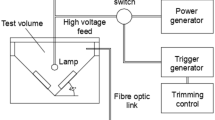
PL involves the use of intense pulses of short duration and a broad spectrum to ensure microbial inactivation on the surface of either foods or packaging materials. Electromagnetic energy is accumulated in a capacitor during fractions of a second and then released in the form of light within a short time (nanoseconds to milliseconds), resulting in an amplification of power with a minimum of additional energy consumption (Dunn et al. 1995). Typically, equipment used to produce PL is composed of one or more adjustable xenon lamp units, a power unit, and a high-voltage connection that allows the transfer of a high current electrical pulse. As the current passes through the gas chamber of the lamp unit, a short, intense burst of light is emitted. The light produced by the lamp includes broad spectrum wavelengths from UV to near-infrared. The wavelength distribution ranges from 100 to 1,100 nm: UV (100–400 nm), visible light (400–700 nm), and infrared (700–1,100 nm). Pulses of light used for food processing applications typically emit 1 to 20 flashes per second at an energy density in the range of about 0.01 to 50 J cm−2 at the surface (Barbosa-Canovas et al. 1998).
Practical UV disinfection systems, as applied for instance to water treatments, have traditionally used low or medium pressure mercury lamps as the source of germicidal radiation. Low pressure mercury lamps are considered to provide monochromatic radiation at 254 nm, whereas germicidal wavelength range of medium pressure mercury UV lamps is between 200 and 300 nm. The UV radiation from both types of mercury lamps is emitted continuously. Continuous UV light has several disadvantages, such as poor penetration depth and low emission power, whereas PL sterilization has comparatively higher penetration depth and emission power. PL treatment is more effective and rapid for microorganism inactivation than continuous UV light, because the energy is multiplied manifold (Food and Drug Administration 2000; Dunn et al. 1995). Power emission from a continuous UV light system ranged from 100 to 1,000 W (Demirci 2002); however, a PL system can produce a peak power distribution as high as 35 MW (MacGregor et al. 1997; McDonald et al. 2000). Moreover, the PL may reduce the temperature build-up compared with continuous UV light due to short pulse duration and cooling period between pulses (Krishnamurthy et al. 2004).
Microbial Inactivation by PL
The efficiency of PL on inactivating bacteria, mold spores, and viruses is well documented (MacGregor et al. 1997; Rowan et al. 1999; Anderson et al. 2000; Roberts and Hope 2003). The effects of PL on microorganisms in vitro are shown in Table 1. Krishnamurthy et al. (2004) investigated the use of PL treatments to inactivate Staphyloccocus aureus in a buffer solution as well as in agar-seeded plates. They found 7 to 8 log CFU mL−1 reduction of S. aureus on suspended and agar-seeded cells treated at 5.6 J cm−2 per pulse for 5 s without a substantial increase in temperature. Concerning the effect of PL on molds, it was reported that about 4.8 log cycles of Aspergillus niger spore inactivation resulted with 5 pulsed light flashes at 1 J cm−2 (Wekhof et al. 2001). PL treatment was effective in the inactivation of poliovirus and adenovirus. Although both viruses showed susceptibility to the treatment, adenoviruses were more resistant to PL than polioviruses. Log reductions of 4 CFU mL−1 in poliovirus were observed with 10 pulses corresponding to a dose of 12 mJ cm−2, while the same number of pulses resulted in approximately 1 log reduction of adenovirus (Lamont et al. 2007).
Microbial inactivation by exposure to PL is attributed to the effect of the broad spectrum UV content and the energy density applied with the treatment, which in turn related with the pulse width and the high peak power of the pulse. The composition of the emitted spectrum of the PL source affects significantly the effectiveness of the treatment (Marquenie et al. 2003a, b). Exposure to UV short wavelengths between 100 and 280 nm (UV-C) has been used in a broad range of antimicrobial applications including disinfection of water, air, surfaces, and food (Shama 1999; Bintsis et al. 2000; Wright et al. 2000; Koutchma et al. 2004). On a polyethylene terephthalate (PET) surface, the inactivation of A. niger spores by PL decreased three- to fivefold by eliminating UV-C from the spectrum of light pulses (Wekhof et al. 2001). PL sources of low UV intensities were not effective in reducing microbial populations (Anderson et al. 2000). Rowan et al. (1999) reported that the inactivation of food-related microorganims such as Listeria monocytogenes, Escherichia coli, Salmonella enteritidis, Pseudomonas aeruginosa, Bacillus cereus, and Staphylococcus aureus after 200 pulses was of 2 and 6 log CFU mL−1 using low and high UV content, respectively. Wang et al. (2005) used a pulsed xenon flash lamp with a monochromator to investigate the wavelength sensitivity of E. coli to inactivation by the pulsed UV light. These authors reported that the germicidal efficiency at 254 nm (0.33 log CFU mL−1 per mJ/cm2) obtained with the xenon flash lamp shows no obvious difference to the published data using continuous UV low pressure mercury lamps at the same wavelength. However, these authors suggested that the short pulse width and high doses of the pulsed UV source may provide some practical advantages over continuous UV sources in those situations where rapid disinfection is required. High energy delivered to the lamp produces an intense pulse of light, which typically lasts a few hundred microseconds. This can result in the application of lethal UV dose levels that would require continuous UV sources to operate over a much longer time period. As what occurs with conventional continuous UV light, the main mechanism of microbial inactivation by PL is explained through the photochemical effect (Wang et al. 2005), which consists in the formation of pyrimidine dimers in the DNA of bacteria, viruses, and other pathogens, thus preventing the cell from replicating (Rowan et al. 1999). However, additional modes of inactivation such as photothermal and photophysical effects have been proposed (Wuytack et al. 2003; Krishnamurthy et al. 2007).
Some authors have attributed the cell disruption to a photothermal effect caused by absorption of UV light when fluence, energy received by the sample, is excessive (Hiramoto 1984; Wekhof 2000; Wekhof et al. 2001). Wekhof (2000) measured the temperature of E. coli cells on a polymeric surface when exposed to PL in different conditions and found that no temperature increase was detected until a fluence threshold was exceeded, after which temperature rapidly rose up to values higher than 120 °C. Fine and Gervais (2004) have recently used the term ‘mean energy level threshold’ to identify a fluence level beyond which the inactivation is dramatically increased. Wekhof et al. (2001) attributed A. niger inactivation on a PET surface at low fluence values, around 1–3 J cm−2, to the photochemical effect of UV-C light, while the contribution of photothermal action of UV-A (long wave, λ = 315–400 nm) and UV-B (medium wave, λ = 280–315 nm) light seemed to be more relevant at high fluence values of 5–6 J cm−2. Takeshita et al. (2003) observed that the DNA damage in S. cerevisiae cells after a continuous UV light treatment was slightly higher than after a PL treatment. However, protein elution from yeast cells after PL irradiation was higher than that observed under continuous UV irradiation when increasing fluence. After a dose of 1.4 J cm−2, vacuole expansion and cell membrane distortion were observed in the case of yeast cells exposed to PL. Thus, microbial inactivation by PL would be mainly due to structural changes in DNA although the cell could be disintegrated after an instantaneous overheating of the cellular constituents (Wekhof 2003). According to Fine and Gervais (2004), this overheating may cause vaporization and generate a small steam flow to cause membrane destruction. Krishnamurthy et al. (2008) also suggested photophysical effects of PL on S. aureus in phosphate buffer treated for 5 s, caused by disturbances of intermittent high energy pulses, since temperature increase during treatment was negligible (2 °C). S. aureus exhibited cell wall damage, cytoplasmic membrane shrinkage, cellular content leakage, and mesosome disintegration based on transmission electron microscopy and Fourier transform infrared spectroscopy observations.
The photochemical effects of PL on some living beings, including microorganisms, can be reversed by illumination with longer wavelengths, especially visible light, a repairing mechanism called photoreactivation (McDonald et al. 2000). Photoreactivation in flashed cells was evident after PL treatment (Gómez-López et al. 2005a), the rate of this photoreactivation after a PL treatment was slower than after a continuous UV treatment (Otaki et al. 2003). In fact, some authors have taken the precaution of wrapping the Petri dishes in aluminum foil to avoid photoreactivation after PL treatments (MacGregor et al. 1997; Rowan et al. 1999; Anderson et al. 2000).
Microorganisms have been shown to differ in their sensitivity to PL. Gram-positive bacteria, such as B. cereus, have been shown to be more resistant to the effects of PL than Gram-negative bacteria, such as S. enteritidis and E. coli (Anderson et al. 2000). These authors suggested that the variation in PL sensitivity by the microorganisms may be related to differences in bacterial cell wall composition as well as due to their protective and repair mechanisms against the damage. The fungal spores, A. niger and Fusarium culmorum, have been shown to display a greater resistance to PL treatment compared with the tested bacteria such as E. coli, S. enteritidis, or B. cereus (Anderson et al. 2000). According to these authors, the extent of the fungal spore resistance of A. niger could be attributed to the presence of protective dark pigments in the wall layers that surround the spore form. An absorbance scan of pigment extracts from the fungus over the range 240–480 nm indicated that the pigments of A. niger absorb strongly in the UV range. This UV absorbing characteristic would appear to play a significant role in the defense mechanism of this organism against the deleterious effects of PL. On the other hand, Turtoi and Nicolau (2007) believed that dark colored fialospores produced by A. niger (black) and Aspergillus cinnamomeus (brown) could absorb more light energy and thus, be destroyed faster by PL than those of Aspergillus repens (green). These authors also reported that blastospores produced by Cladosporium herbarum spores are more easily inactivated (0.795 J cm−2) than fialospores produced by aspergilli (0.81–0.927 J cm−2).
The combination of a PL treatment for 120 s and continuous UV radiation at doses of 0.10 J cm−2 had a synergistic effect on the inactivation of conidia from Botrytis cinerea and Monilia fructigena. Differences between both fungi were attributed to the greater sensitivity of M. fructigena conidia to UV light. A continuous UV dose of 0.10 J cm−2 caused almost complete inactivation of M. fructigena conidia, so that additional inactivation by PL could not be measured (Marquenie et al. 2003a). This study also demonstrated a synergistic effect of the combination of a thermal (35–45 °C for 3–15 min) and a PL treatment, although complete inactivation of conidia was not observed. According to these authors, a thermal treatment could possibly inhibit DNA repair mechanisms after damage caused by the light exposure treatments, thus improving the effectiveness of the PL treatments.
Process Critical Factors
When designing a PL treatment for food commodities, the number of pulses, distance from the source of light, and thickness of the product are critical parameters for process optimization, in order to maximize the effectiveness against microorganisms and to minimize product alteration. Changes in food attributes can be attributed to thermal damage caused by the use of high fluencies. In general, temperature increase of products exposed to PL is much lower and localized in a thinner surface layer than that of an equivalent continuous UV light treatment, due to the short duration of pulses. Dunn et al. (1989) found that in hard crusted bread roll samples treated with pulses of 16 J cm−2, the surface temperature increase was negligible when using 1 pulse and about 5 °C when using 2 pulses. Nevertheless, when the light intensity or treatment duration is relatively high, the temperature increase of the product may be greater than desirable, causing burning of surface layers of food. This was confirmed by Hillegas and Demirci (2003) who treated samples of clover honey with a large number of 5.6 J cm−2 pulses and observed a sample surface temperature increase from 20 to up 80–100 °C when exposed to more than 50–100 pulses. Without an efficient cooling system incorporated in the equipment, PL treatments for long treatment times can seriously compromise quality due to an excessive temperature increase (Elmnasser et al. 2007).
Energy incidence is affected by the distance from light source to the sample. Distance clearly influences the inactivation efficacy of PL: the longer the distance between the sample and the lamp, the lower the lethality of the process (Gómez-López et al. 2005a). Sharma and Demirci (2003) and Jun et al. (2003) proposed a quadratic regression model by using a response surface methodology to model the effect of distance from the UV strobe on the level of microbial inactivation. The position and orientation of the lights in an industrial decontamination unit can also affect the energy incidence on the product. Gómez-López et al. (2005a) demonstrated that a group of food pieces placed very close to the lamp was not efficiently decontaminated.
The efficiency of PL has shown to be influenced by inoculum size when the treatment is applied to surfaces (Uesugi et al. 2007). Gómez-López et al. (2005a) observed a strong decrease in inactivation efficiency when high counts of L. monocytogenes were reached on the surface of an agar medium. Microorganisms placed in the upper layers will become inactivated, but they will shadow the rest from the light. In the case of foods, a partial disinfection could be observed due to shielding effects of highly directional coherent PL sources. Microorganisms may reside in crevices or in irregularities (injuries) of the food surface or may penetrate under the product epidermis, thus decreasing the efficiency of the treatments and requiring a larger PL exposure (Lagunas-Solar et al. 2006). Seo and Frank (1999) used confocal scanning electron microscopy to observe that viable E. coli 0157:H7 cells are able to be attached to the surface, trichomes, and stomata leaves and accumulate in the stomata of cut lettuce. They observed that viable E. coli 0157:H7 cells are able to penetrate into cut leaf edges to a depth of 20 μm. For maximum disinfection efficiency, Lagunas-Solar et al. (2006) suggested multidirectional incident beams and random movement of products for uniform surface exposition.
The effect of product thickness on PL-induced microbial inactivation was studied by Hillegas and Demirci (2003). They showed that a PL treatment of 135 pulses of 5.6 J cm−2 caused a 39.5% reduction of Clostridium sporogenes in 2-mm thick clover honey samples, while it had no effect in 8-mm samples. The application of 405 pulses at 5.6 J cm−2 resulted in 73.9% reduction in the thinner samples and 14.2% reduction in the thicker ones. In these latter samples, at least 540 pulses were required to achieve almost 1 log reduction of C. sporogenes. Similar results were obtained by Tonon and Agoulon (2003) in milk. In samples treated with 4 pulses of 6 J cm−2, the initial bacterial population was reduced to 28% at a depth of 1 mm and 43% at a depth of 2 mm, while no bacterial reduction was observed at depth greater than 4 mm.
Furthermore, every food product, liquid or solid, has its own composition, and this may determine the effect of the PL treatment. Certain food components could absorb the effective wavelengths and decrease the efficiency of the treatment. PL treatment was not effective in inactivation of L. monocytogenes, P. phosphoreum, and C. lambica on proteinaceous or oily foods, whereas foods high in carbohydrates such as fruits and vegetables seem to be more suitable for this treatment (Gómez-López et al. 2005b). These authors observed that the decontamination effect of PL treatment of about 1.5 log CFU cm−2 for C. lambica was totally reduced in the presence of 10% oil or 10% casein. Part of the PL could have been absorbed by proteins and oils, decreasing the effective radiation dose on microorganisms (Gómez-López et al. 2005b).
Effects of PL on Food Products
Table 2 summarizes the most relevant results regarding microbial inactivation by PL treatments in foods, beverages, packaging materials, and food contact surfaces.
Liquid Foods
Many fluids, such as water, have a high degree of transparency to a broad range of wavelengths including visible and UV light, while other liquids, such as sugar solutions and wines, exhibit a more limited transparency. Increasing the amount of solids will diminish the intensity of penetration of the UV radiation (Shama 1999; Bintsis et al. 2000). In an aqueous solution, the lower the transparency, the less effective the PL treatment (Tonon and Agoulon 2003). Liquids with high UV absorbance must be treated as a thin layer in order to reduce radiation absorption by the liquid (Wright et al. 2000). In this manner, UV absorption by the liquid is low and bacteria are more likely to be subjected to lethal doses (Shama 1992). The absorbance of clarified fresh juices and juices containing pulp varies considerably. Clarified apple juice has a low absorbance, with absorption coefficients about 11 cm−1, whereas absorbance of orange juice can achieve values close to 50 cm−1 (Koutchma et al. 2004). In addition, a positive correlation between vitamin C content and the absorption coefficient of clear apple juices was observed (Koutchma 2008).
Milk was efficiently cold pasteurized by the exposure to PL at a minimum dose of 12.6 J cm−2 delivered in 56 s (Smith et al. 2002). Complete inactivation of S. aureus was obtained when processing milk in a continuous system applying PL (Krishnamurthy et al. 2007). These authors concluded that a proper design of a PL system for continuous flow milk pasteurization requires reduced treatment times to avoid an excessive increase in temperature, which may cause some changes in the quality of the milk. Quality and sensory changes during PL treatments have not been extensively studied yet. Compared to continuous UV light, PL can effectively limit oxidative reactions because of the short pulse duration, typically 300 ns to 1 ms (Fine and Gervais 2004). PL treatment seems not to affect protein or lipid components of milk. Elmnasser et al. (2008) did not observe changes in amino acid composition of proteins and lipid oxidation after milk treatment.
PL treatment is more effective for the sterilization of surfaces than liquid media. The efficacy of PL (5.6 J cm−2 per pulse) for the inactivation of S. aureus in agar or liquid media was investigated by Krishnamurthy et al. (2004). They observed 5.0 and 1.35 log reductions in agar-seeded cells and suspended cells, respectively, after a 1-s treatment. As the sample depth of suspended cells increases, the inactivation level of S. aureus decreases because of the poor penetration capacity of PL. The effectiveness of PL on the inactivation of suspended cells in liquid mediums could be increased by minimizing the sample depth, reducing distance from the sample and/or increasing the treatment time. There is a need for optimizing the experimental parameters to achieve the target inactivation level for specific applications. Studies carried out by Uesugi et al. (2007) demonstrated that PL is capable to deliver the same level of microbial reduction in clear liquids, regardless of the level of contamination. According to these authors, the Weibull model is adequate to accurately predict microbial inactivation in clear liquids, but it fails for products where the influence of various substrate properties on inactivation is significant.
Solid Foods of Vegetable Origin
The log reductions achieved after treating minimally processed vegetables such as spinach, celery, green paprika, soybean sprouts, radicchio, carrot, iceberg lettuce, and white cabbage with PL of 7 J pulse intensity were between 0.21 and 1.67 at 45 s/side, and between 0.56 and 2.04 at 180 s/side (Gómez-López et al. 2005b). The presence of off-odors limited the shelf-life of shredded white cabbage stored under MAP at 7 °C up to 9 days, whereas overall visual quality limited that of shredded lettuce up to 3 days. The results of this study also indicate that PL treatment induced an 80% increase in the respiration rate of lettuce, whereas the respiratory rate of cabbage was not affected. Reductions in aerobic counts, from 1.6 log CFU mL−1 for carrots to more than 2.6 log CFU mL−1 for paprika, were reported by Hoornstra et al. (2002), using only 2 pulses of 0.15 J cm−2 per flash. According to these authors, 2 log reductions almost extended the shelf-life of cut vegetables at 7 °C by about four additional days. Jun et al. (2003) used a response surface methodology to determine the optimal processing conditions to the inactivation of A. niger spores in corn meal. Thus, the model predictions indicated that maximum 4.93 log reduction could be obtained when the treatment time was 100 s at a distance from the strobe of 3 cm and a maximum energy of 5.6 J cm−2 per pulse. Sharma and Demirci (2003) achieved a substantial reduction of 4.89 log CFU mL−1 in the population of E. coli O157:H7 on a 6.25-mm thick layer of alfalfa seeds treated with 5.6 J cm−2 for 90 s (270 pulses) at 8 cm distance, but only 1.42 log reductions at 13 cm. For colored food powders (black pepper and wheat flour), the thermal effect of PL dominated the UV effect. Visual and flavor qualities of food powders were significantly altered before the decontamination threshold of 58 J cm−2 was reached. Color changes of black pepper and wheat flour were attributed to overheating combined with oxidation, which occurred clearly more rapidly for black pepper than for wheat flour since dark products absorb more light energy than lighter products (Fine and Gervais 2004). On the other hand, 2–5 flashes of PL at a fluence of 3 J cm−2 retained the color of potato slices after a prolonged storage, while the untreated samples began rapidly to brown (Dunn et al. 1989).
PL has been also studied to evaluate the surface disinfection of fresh fruits (Lagunas-Solar et al. 2006; Bialka and Demirci 2007, 2008). Microorganisms tested by Lagunas-Solar et al. (2006), Alternaria alternate, A. niger, B. cinerea, Fusarium oxysporum, Fusarium roseum, Monilinia fructicola, Penicillium expansum, Penicillium digitatum, and Rhizopus stolonifer, were completely or partially killed after exposure of fruit surfaces to PL (248-nm) treatments. With the exception of A. niger, most fungi were controlled with less than 0.5 J cm−2 on fruit surfaces. A niger required 1.9 J cm−2 due to the presence of highly UV absorbent melanin-type pigments. These authors observed that the energy threshold that causes injury in fruits such as apples, oranges, lemons, peaches, raspberries, and table grapes was below 2 J cm−2.
After a PL treatment for decontaminating blueberries, maximum 4.3 and 2.9 log CFU mL−1 reductions were achieved at 22.6 J cm−2 after a 60-s treatment for Salmonella and E. coli O157:H7, respectively (Bialka and Demirci 2007). On raspberries and strawberries, maximum 3.9 and 2.1 log reductions of E. coli O157:H7 were obtained at 72 and 25.7 J cm−2, respectively, while 3.4 and 2.8 log reductions of Salmonella were observed at 59.2 and 34.2 J cm−2. Moreover, the inactivation of E. coli O157:H7 and Salmonella by PL was accurately estimated by the Weibull model rather than by first-order kinetics (Bialka et al. 2008). Marquenie et al. (2003b) found no suppression of fungal development by treating B. cinerea inoculated on strawberries up to 250 s with a pulse intensity of 7 J. In addition, the combination of PL with short thermal treatments (40–45 °C for 3–15 min) or with continuous UV-C irradiation did not result in a significant decrease in fungal growth, although the lag period was increased by 1 or 2 days.
It has also been shown that exposure to low doses of continuous UV light increases the resistance of a fruit to pathogens. In treated plant tissues, chemical species with beneficial effects on human health can be induced (Lagunas-Solar et al. 2006; Shama 2007; Bialka and Demirci 2008). These substances include phytoalexins such as scoparone in oranges (Dhallewin et al. 1999), 6-methoxymellein in carrots (Mercier et al. 2000), and resveratrol in grapes, with a number of cardioprotective properties (Cantos et al. 2002). Enzymes such as chitinases and glucanases in peaches (El Ghaouth et al. 2003) and tomatoes are also induced (Barka 2001). It has also been claimed that treatment with hormetic doses of UV results in an enhancement in the levels of anthocyanins in strawberries (Baka et al. 1999) and apples (Dong et al. 1995). However, an induced physiological reaction was not observed in PL-treated strawberries, since the UV-C component in the emitted light could probably be too small to induce this response (Marquenie et al. 2003b). In cut fruits, wound responses also seem to be altered due to UV radiation. A hypersensitive defense response in fresh-cut cantaloupe melon was induced by a continuous UV-C treatment, resulting in increased accumulation of POD. In addition, the results showed that reduced lipase activity in UV-C-treated samples during storage and other induced antisenescence defense responses appeared to reduce rancidity and improve firmness retention in the stored fruit (Lamikanra et al. 2005).
An approach to the development of a peanut product with possibly lower allergenic properties through PL treatment was carried out by Chung et al. (2008). As it was demonstrated by SDS-PAGE, bands corresponding to peanut allergens (63 kDa) were missing in the pulsed treated peanut extracts and liquid peanut butter.
Other Foods
As reported by Dunn et al. (1995), PL treatments achieved high levels of microbial inactivation on relatively simple surfaces, while generally showed only 1–3 log reductions on complex surfaces such as meats. Part of the radiation may have been absorbed by proteins and lipids, thus decreasing the effective radiation dose on microorganisms (Gómez-López et al. 2005b). Proteins have strong absorption of UV at about 280 nm as well as at higher wavelengths of the UV-B region, while lipids with isolated or conjugated double bonds also absorb UV (Hollósy 2002). Dunn et al. (1997) demonstrated that beef steaks treated with PL using 5 J cm−2 to each side and stored 3 days at 4–5 °C exhibited 2 log reductions in microbial counts. Listeria innocua was reported to be reduced by 2 log cycles on hot dogs after a PL treatment (Dunn et al. 1995). Their results also showed that shrimp treated with PL and stored under refrigeration for 7 days remained edible, while untreated shrimp exhibited extensive microbial degradation. In another study, Ozer and Demirci (2006) demonstrated that 1 log reduction of E. coli O157:H7 or L. monocytogenes after a PL treatment (5.6 J cm−2 per pulse) could be achieved after 60 s treatment at 8 cm distance without affecting the quality of salmon fillets. At shorter distances such as 3 and 5 cm, samples were overheated when treatment times increased up to 30 and 45 s, respectively, with consequent visual color and quality changes.
Regarding nutritional analysis carried out by Dunn et al. (1995), frankfurters exposed to up to 30 J cm−2 of PL did not exhibit differences in protein, riboflavin, nitrosamine, benzopyrene, and vitamin C content compared to untreated samples. While a strong loss of riboflavin is observed in foods because of heat, oxygen, and light, Dunn et al. (1995) reported that even PL treatments did not influence riboflavin concentration in beef, chicken, and fish. Few data are available related to the effects of PL treatments on sensory properties of foods. Dunn et al. (1989) reported in their US patent that no visible discoloration and no changes in taste were caused by 1–2 pulses of 2 J cm−2 in dry cottage cheese. Shuwaish et al. (2000) found that neither Hunter color nor shear force values significantly changed in pre-packaged catfish fillets treated by 2–4 pulses of 2.5–5 J cm−2.
Packaging Materials and Food Contact Materials
PL technology is applicable to sterilize or reduce microbial population of packaging material surfaces or food contact materials in processing plants (Dunn 1996). On the surface of different packaging materials inoculated with 10–1,000 CFU cm−2, a single pulse of 1.25 J cm−2 inactivated S. aureus, while B. cereus and Aspergillus spp. spores were inactivated with intensities greater than 2 J cm−2 (Dunn et al. 1991). McDonald et al. (2000) reported almost identical inactivation levels of Bacillus subtilis for the decontamination of surfaces with 4.10−3 J cm−2 PL and 8.10−3 J cm−2 continuous UV light treatments. Molds that are frequently found on the surface of food packaging papers, such as A. niger, A. repens, A. cinnamomeus, and C. herbarum, were significantly reduced on paper-polyethylene packaging material after a PL treatment (Turtoi and Nicolau 2007). Inactivation of C. herbarum exposed to 0.977 J cm−2 for 30 ms was of about 2.7 log reduction, which is more than enough for a usual contaminated packaging material.
Food contact surface properties have been shown to influence the microbicidal effect of PL. Woodling and Moraru (2005) inoculated with L. innocua four types of stainless steel surfaces, which were treated with 1.27 J cm−2 pulses. Their results demonstrate that surface topography has a complex influence on the efficacy of PL treatments for surface microbial reduction. Thus, the effects of PL on microbial inactivation decreased in the smoothest finish due to its highly hydrophobic and reflective nature that leads to cell clustering. On the other hand, the roughest surface allowed for a relatively uniform distribution of the cells on the treated surface although it also allowed some hiding of microbial cells, which shielded the cells from the full effect of the PL treatment.
Potential Applications of PL Technology and Main Limitations
PL has potential applications for the treatment of foods that require a rapid disinfection. Other advantages of PL are the lack of residual compounds and the absence of applied chemicals disinfectants and preservatives. Foods with smooth surfaces such as fresh whole fruit and vegetable commodities, hard cheeses, or smooth surface meat slices are suitable for treatment with PL where surface contamination is a concern for microbial contamination. However, there are nowadays few commercial companies producing disinfection systems based on PL. This technology can be used in the final steps of minimal processing; however, treatments that effectively penetrate packaging materials are still being a challenge to this technology. Gómez-López et al. (2007) and Elmnasser et al. (2007) summarized the main limitations to the PL systems for food applications. According to them, one of the most important challenges to PL is its limited efficacy for controlling food heating. Heating limited the treatment of alfalfa seeds (Sharma and Demirci 2003), grated carrots (Gómez-López et al. 2005b), and raw salmon fillets (Ozer and Demirci 2006). In food powders (black pepper and wheat flour), the thermal effect of PL resulted in undesirable color alterations of the products before microbial inactivation was completed (Fine and Gervais 2004). Limited PL efficiency because of the shadow effect has also been observed on food products. The shading effect will reduced the effective radiation dose available for microbial inactivation. Foods with rough or uneven surfaces, crevices, or pores are unsuitable for PL because of the ability of microorganisms to harbor in small openings. PL is not an adequate technology for cereals, grains, and spices due to their opaque nature, whereas it is an effective method of decontaminating packaging materials.
Conclusions
PL is a novel nonthermal technology to inactivate pathogenic and spoilage microorganisms on foods. The significant microbial reductions in very short treatment times, the limited energy cost of PL, the lack of residual compounds, and its great flexibility are some of the major benefits of the technique. This method is clearly efficient to inactivate microorganisms in vitro, but its potential on real foods is still under investigation. Further studies need to be conducted to assess the effects of PL treatments on food properties beyond safety and spoilage. There is a need for optimizing the critical process factors to achieve the target inactivation level for specific food applications without affecting quality. PL equipment with good penetration and short treatment times need to be designed for commercial purposes. In addition, the applicability of PL treatments on an industrial scale needs to be compared with other nonthermal or conventional thermal processes.
References
Anderson, J. G., Rowan, N. J., MacGregor, S. J., Fouracre, R. A., & Farish, O. (2000). Inactivation of food-borne enteropathogenic bacteria and spoilage fungi using pulsed-light. IEEE Transactions on Plasma Science, 28(1), 83–88. doi:10.1109/27.842870.
Baka, M., Mercier, J., Corcruff, R., Castaigne, F., & Arul, J. (1999). Photochemical treatment to improve storability of fresh strawberries. Journal of Food Science, 64, 1068–1072. doi:10.1111/j.1365-2621.1999.tb12284.x.
Barbosa-Canovas, G. V., Pothakamury, U. R., Palou, E., & Swanson, B. G. (1998). Nonthermal preservation of foods pp. 139–161. New York: Marcel Dekker.
Barka, E. A. (2001). Protective enzymes against reactive oxygen species during ripening of tomato (Lycopersicon esculentum) fruits in response to low amounts of UV-C. Australian Journal of Plant Physiology, 28, 785–791.
Bialka, K. L., & Demirci, A. (2007). Decontamination of Escherichia coli O157:H7 and Salmonella enterica on blueberries using ozone and pulsed UV-light. Journal of Food Science, 72(9), 391–396. doi:10.1111/j.1750-3841.2007.00517.x.
Bialka, K. I., & Demirci, A. (2008). Efficacy of pulsed UV-light for the decontamination of Escherichia coli O157:H7 and Salmonella enterica on raspberries and strawberries. Journal of Food Science, 00(0), 1–7.
Bialka, K. I., Demirci, A., & Purl, V. M. (2008). Modelling the inactivation of Escherichia coli O157:H7 and Salmonella enterica on raspberries and strawberries resulting form exposure to ozone or pulsed UV-light. Journal of Food Engineering, 85(3), 444–449. doi:10.1016/j.jfoodeng.2007.08.007.
Bintsis, T., Litopoulou-Tzanetaki, E., & Robinson, R. (2000). Existing and potential applications of ultraviolet light in the food industry—A critical review. Journal of the Science of Food and Agriculture, 80, 637–645. doi:10.1002/(SICI)1097-0010(20000501)80:6<637::AID-JSFA603>3.0.CO;2-1.
Cantos, E., Espin, J. C., & Tomas-Barbaran, F. A. (2002). Postharvest stilbene enrichment of red and white table grape varieties using UVC irradiation pulses. Journal of Agricultural and Food Chemistry, 50, 6322–6329. doi:10.1021/jf020562x.
Chung, S. Y., Yang, W., & Krishnamurthy, K. (2008). Effects of pulsed UV-light on peanut allergens in extracts and liquid peanut butter. Journal of Food Science, 73(5), 400–404. doi:10.1111/j.1750-3841.2008.00784.x.
Demirci, A. (2002). Novel processing technologies for food safety. Association of Food Drug Officials, 64(4), 1–8.
Dhallewin, G., Schirra, M., Manueddu, E., Piga, A., & Ben-Yehoshua, S. (1999). Scoparone and scopoletin accumulation and ultraviolet-C induced resistance to postharvest decay in oranges as influenced by harvest date. Journal of the American Society for Horticultural Science, 124, 702–707.
Dong, Y. H., Mitra, D., Kootstra, A., Lister, C., & Lancaster, J. (1995). Postharvest stimulation of skin color in Royal-gala apple. Journal of the American Society for Horticultural Science, 120, 95–100.
Dunn, J. (1996). Pulsed light and pulsed electric field for foods and eggs. Poultry Science, 75(9), 1133–1136.
Dunn, J., Bushnell, A., Ott, T., & Clark, W. (1997). Pulsed white light food processing. Cereal Foods World, 42, 510–515.
Dunn, J. E., Clark, R. W., Asmus, J. F., Pearlman, J. S., Boyer, K., Painchaud, F., et al. (1989). Methods for preservation of foodstuffs. US Patent number 4871559.
Dunn, J. E., Clark, R. W., Asmus, J. F., Pearlman, J. S., Boyer, K., Painchaud, F., et al. (1991). Methods for preservation of foodstuffs. US patent number 5034235.
Dunn, J., Ott, T., & Clark, W. (1995). Pulsed light treatment of food and packaging. Food Technologist, 49(9), 95–98.
El Ghaouth, A., Wilson, C. L., & Callahan, A. M. (2003). Induction of chitinase, beta-1,3-glucanase, and phenylalanine ammonia lyase in peach fruit by UV-C treatment. Phytopathology, 93, 349–355. doi:10.1094/PHYTO.2003.93.3.349.
Elmnasser, N., Guillou, S., Leroi, F., Orange, N., Bakhrouf, A., & Federighi, M. (2007). Pulsed-light system as a novel food decontamination technology: A review. Canadian Journal of Microbiology, 53, 813–821. doi:10.1139/W07-042.
Elmnasser, N., Dalgalarrondo, M., Orange, N., Bakhrouf, A., Haertlé, T., Federighi, M., et al. (2008). Effect of pulsed-light treatment on milk proteins and lipids. Journal of Agricultural and Food Chemistry, 56, 1984–1991. doi:10.1021/jf0729964.
Fine, F., & Gervais, P. (2004). Efficiency of pulsed UV light for microbial decontamination of food powders. Journal of Food Protection, 67, 787–792.
Food and Drug Administration. (2000). Kinetics of microbial inactivation for alternative food processing technologies: pulsed light technology. Available at: http://vm.cfsan.fda.gov/∼comm/ift-puls.html. Accessed 2 May 2008.
Gómez-López, V. M., Devlieghere, F., Bonduelle, V., & Debevere, J. (2005a). Factors affecting the inactivation of microorganisms by intense light pulses. Journal of Applied Microbiology, 99, 460–470. doi:10.1111/j.1365-2672.2005.02641.x.
Gómez-López, V. M., Devlieghere, F., Bonduelle, V., & Debevere, J. (2005b). Intense light pulses decontamination of minimally processed vegetables and their shelf-life. International Journal of Food Microbiology, 103, 79–89. doi:10.1016/j.ijfoodmicro.2004.11.028.
Gómez-López, V. M., Ragaert, P., Debevere, J., & Devlieghere, F. (2007). Pulsed light for food decontamination: A review. Trends in Food Science & Technology, 18, 464–473. doi:10.1016/j.tifs.2007.03.010.
Guerrero-Beltrán, J. A., & Barbosa-Cánovas, G. V. (2004). Review: Advantages and limitations on processing foods by UV light. Food Science and Technology International, 10, 137–147. doi:10.1177/1082013204044359.
Hillegas, S. L., & Demirci, A. (2003). Inactivation of Clostridium sporogenes in clover honey by pulsed UV-light treatment. Agricultural Engineering International, V. Manuscritp FP 03 009.
Hiramoto T. (1984). Method of sterilization. US Patent number 4464336.
Hollósy, F. (2002). Effects of ultraviolet radiation on plant cells. Micron, 33, 179–197. doi:10.1016/S0968-4328(01)00011-7.
Hoornstra, E., de Jong, G., & Notermans, S. (2002). Preservation of vegetables by light. In Conference frontiers in microbial fermentation and preservation (pp. 75–77). The Netherlands: Wageningen.
Jun, S., Irudayaraj, J., Demirci, A., & Geiser, D. (2003). Pulsed UV-light treatment of corn meal for inactivation of Aspergillus niger spores. International Journal of Food Science & Technology, 38, 883–888. doi:10.1046/j.0950-5423.2003.00752.x.
Koutchma, T. (2008). UV light for processing foods. Ozone: Science & Engineering, 30, 93–98. doi:10.1080/01919510701816346.
Koutchma, T., Keller, S., Parisi, B., & Chirtel, S. (2004). Ultraviolet disinfection of juice products in laminar and turbulent flow reactors. Innovative Food Science & Emerging Technologies, 5, 179–189. doi:10.1016/j.ifset.2004.01.004.
Krishnamurthy, K., Demirci, A., & Irudayaraj, J. (2004). Inactivation of Staphylococcus aureus by pulsed UV-light sterilization. Journal of Food Protection, 67, 1027–1030.
Krishnamurthy, K., Demirci, A., & Irudayaraj, J. M. (2007). Inactivation of Staphylococcus aureus in milk using flow-through pulsed UV-light treatment system. Journal of Food Science, 72(7), M233–M239. doi:10.1111/j.1750-3841.2007.00438.x.
Krishnamurthy, K., Tewari, J. C., Irudayaraj, J., & Demirci, A. (2008). Microscopic and spectroscopic evaluation of inactivation of Staphylococcus aureus by pulsed UV light and infrared heating. Food and Bioprocess Technology. doi:10.1007/s11947-008-0084-8.
Lagunas-Solar, M. C., Piña, C., MacDonald, J. D., & Bolkan, L. (2006). Development of pulsed UV light processes for surface fungal disinfection of fresh fruits. Journal of Food Protection, 69(2), 376–384.
Lamikanra, O., Kuenemon, D., Ukuku, D., & Bett-Garber, K. L. (2005). Effect of processing under ultraviolet light on the shelf life of fresh-cut cantaloupe melon. Journal of Food Science, 70(9), C534–C539.
Lamont, Y., Rzezutka, A., Anderson, J. G., MacGregor, S. J., Given, M. J., Deppe, C., et al. (2007). Pulsed UV-light inactivation of poliovirus and adenovirus. Letters in Applied Microbiology, 54(5), 564–567. doi:10.1111/j.1472-765X.2007.02261.x.
MacGregor, S. J., Rowan, N. J., Mcllvaney, L., Anderson, J. G., Fouracre, R. A., & Farish, O. (1997). Light inactivation of food-related pathogenic bacteria using a pulsed power source. Letters in Applied Microbiology, 27, 67–70. doi:10.1046/j.1472-765X.1998.00399.x.
Marquenie, D., Geeraerd, A. H., Lammertyn, J., Soontjens, C., Van Impe, J. F., Michiels, C. W., et al. (2003a). Combinations of pulsed light and UV-C or mild heat treatment to inactivate conidia of Botrytis cinerea and Monilia fructigena. International Journal of Food Microbiology, 85, 185–196. doi:10.1016/S0168-1605(02)00538-X..
Marquenie, D., Michiels, C. W., Van Impe, J. F., Schrevens, E., & Nicolaï, B. N. (2003b). Pulsed white light in combinations with UV-C and heat to reduce storage rot of strawberry. Postharvest Biology and Technology, 28, 455–461. doi:10.1016/S0925-5214(02)00214-4.
McDonald, K. F., Curry, R. D., Clevenger, T. E., Unklesbay, K., Eisenstark, A., & Golden, J. (2000). A comparison of pulsed & continuous ultraviolet light sources for the decontamination of surfaces. IEEE Transactions on Plasma Science, 28, 1581–1587. doi:10.1109/27.901237.
Mercier, J., Roussel, D., Charles, M. T., & Arul, J. (2000). Systemic and local responses associated with UV- and pathogen-induced resistance to Botrytis cinerea in stored carrot. Phytopathology, 90, 981–986. doi:10.1094/PHYTO.2000.90.9.981.
Otaki, M., Okuda, A., Tajima, K., Iwasaki, T., Kinoshita, S., & Ohgaki, S. (2003). Inactivation differences of microorganisms by low pressure UV and pulsed xenon lamps. Water Science and Technology, 47, 185–190.
Ozer, N. P., & Demirci, A. (2006). Inactivation of Escherichia coli O157:H7 and Listeria monocytogenes inoculated on raw salmon fillets by pulsed UV-light treatment. International Journal of Food Science & Technology, 41, 354–360. doi:10.1111/j.1365-2621.2005.01071.x.
Roberts, P., & Hope, A. (2003). Virus inactivation by high intensity broad spectrum pulsed light. Journal of Virological Methods, 110, 61–65. doi:10.1016/S0166-0934(03)00098-3.
Rowan, N. J., MacGregor, S. J., Anderson, J. G., Fouracre, R. A., McIlvaney, L., & Farish, O. (1999). Pulsed-light inactivation of food-related microorganisms. Applied and Environmental Microbiology, 65, 1312–1315.
Seo, K. H., & Frank, J. F. (1999). Attachment of Escherichia coli 0157:H7 to lettuce leaf surfaces and bacterial viability in response to chlorine treatment as demonstrated by using confocal scanning laser microscopy. Journal of Food Protection, 62(1), 3–9.
Shama, G. (1992). Ultraviolet irradiation apparatus for disinfecting liquids of high ultraviolet absorptivities. Letters in Applied Microbiology, 15, 69–72. doi:10.1111/j.1472-765X.1992.tb00727.x.
Shama, G. (1999). Ultraviolet light. In R. K. Robinson, C. Batt, & P. Patel (Eds.), Encyclopedia of food microbiology—3 (pp. 2208–2214). London: Academic.
Shama, G. (2007). Process challenges in applying low doses of ultraviolet light to fresh produce for eliciting beneficial hormetic responses. Postharvest Biology and Technology, 44, 1–8. doi:10.1016/j.postharvbio.2006.11.004.
Sharma, R. R., & Demirci, A. (2003). Inactivation of Escherichia coli O157:H7 on inoculated alfalfa seeds with pulsed ultraviolet light and response surface modelling. Journal of Food Science, 68, 1448–1453. doi:10.1111/j.1365-2621.2003.tb09665.x.
Shuwaish, A., Figueroa, J. E., Silva, J. L. (2000). Pulsed light treated prepackaged catfish fillets. IFT Annual Meeting, 10–14 June 2000, Dallas, USA.
Smith, W. L., Lagunas-Solar, M. C., & Cullor, J. S. (2002). Use of pulsed ultraviolet laser light for the cold pasteurization of bovine milk. Journal of Food Protection, 65(9), 1480–1482.
Takeshita, K., Shibato, J., Sameshima, T., Fukunaga, S., Isobe, S., Arihara, K., et al. (2003). Damage of yeast cells induced by pulsed light irradiation. International Journal of Food Microbiology, 85, 151–158. doi:10.1016/S0168-1605(02)00509-3.
Tonon, F., & Agoulon, A. (2003). Lumiere pulse, principe et application au cas des solutions liquids. Industries Agro-alimentaires, la conservation de demain, 4e edition, 20 November 2003, Talence, France.
Turtoi, M., & Nicolau, A. (2007). Intense light pulse treatment as alternative method for mould spores destruction on paper-polyethylene packaging material. Journal of Food Engineering, 83, 47–53. doi:10.1016/j.jfoodeng.2006.11.017.
Uesugi, A. R., Woodling, S. E., & Moraru, C. I. (2007). Inactivation kinetics and factors of variability in the pulsed light treatment of Listeria innocua cells. Journal of Food Protection, 70(11), 2518–2525.
Wang, T., MacGregor, S. J., Anderson, J. G., & Woolsey, G. A. (2005). Pulsed ultra-violet inactivation spectrum of Escherichia coli. Water Research, 39, 2921–2925. doi:10.1016/j.watres.2005.04.067.
Wekhof, A. (2000). Disinfection with flash lamps. PDA Journal of Pharmaceutical Science and Technology, 54, 264–276.
Wekhof, A. (2003). Sterilization of packaged pharmaceutical solutions, packaging and surgical tools with pulsed UV light. In: Proceedings of the Second International Congress UV Technologies, 9–11 July 2003, Vienna, Austria.
Wekhof, A., Trompeter, F. J., & Franken, O. (2001). Pulse UV disintegration (PUVD): A new sterilisation mechanism for packaging and broad medical-hospital applications. In: Proceedings of the First International Conference on Ultraviolet Technologies, 14–16 June 2001, Washington, DC, USA.
Woodling, S. E., & Moraru, C. I. (2005). Influence of surface topography on the effectiveness of pulsed light treatment for the inactivation of Listeria innocua on stainless-steel surfaces. Journal of Food Science, 70(7), M345–M351. doi:10.1111/j.1365-2621.2005.tb11478.x.
Wright, J. R., Summer, S. S., Hackney, C. R., Pierson, M. D., & Zoecklein, B. W. (2000). Efficacy of ultraviolet light for reducing Escherichia coli O:157:H7 in unpasteurized apple cider. Journal of Food Protection, 63(5), 563–567.
Wuytack, E. Y., Phuong, L. D. T., Aertsen, A., Reyns, K. M. F., Marquenie, D., De Ketelaere, B., et al. (2003). Comparison of sublethal injury induced in Salmonella enterica serovar typhimurium by heat and by different nonthermal treatments. Journal of Food Protection, 66, 31–37.
Acknowledgment
This work was supported by the Ministerio de Ciencia y Tecnología (Spain) through the Project AGL2006-04775/ALI.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oms-Oliu, G., Martín-Belloso, O. & Soliva-Fortuny, R. Pulsed Light Treatments for Food Preservation. A Review. Food Bioprocess Technol 3, 13–23 (2010). https://doi.org/10.1007/s11947-008-0147-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-008-0147-x