Abstract
Purpose of Review
Rapid advances in imaging of the prostate have facilitated the development of focal therapy and provided a non-invasive method of estimating tumour volume. Focal therapy relies on an accurate estimate of tumour volume for patient selection and treatment planning so that the optimal energy dose can be delivered to the target area(s) of the prostate while minimising toxicity to surrounding structures. This review provides an overview of different imaging modalities which may be used to optimise tumour volume assessment and critically evaluates the published evidence for each modality.
Recent Findings
Multi-parametric MRI (mp-MRI) has become the standard tool for patient selection and guiding focal therapy treatment. The current evidence suggests that mp-MRI may underestimate tumour volume, although there is a large variability in results. There remain significant methodological challenges associated with pathological processing and accurate co-registration of histopathological data with mp-MRI. Advances in different ultrasound modalities are showing promise but there has been limited research into tumour volume estimation. The role of PSMA PET/CT is still evolving and further investigation is needed to establish if this is a viable technique for prostate tumour volumetric assessment.
Summary
mp-MRI provides the necessary tumour volume information required for selecting patients and guiding focal therapy treatment. The potential for underestimation of tumour volume should be taken into account and an additional margin applied to ensure adequate treatment coverage. At present, there are no other viable image-based alternatives although advances in new technologies may refine volume estimations in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The aim of focal therapy is to retain equivalent oncological outcomes to whole-gland therapies while reducing the side effects associated with these treatments. Preservation of normal tissue is fundamental to the approach and this relies on an accurate assessment of tumour volume. An accurate knowledge of tumour volume allows maximal therapy to be directed to the target area while minimising damage to the surrounding structures such as neurovascular bundles, bladder neck and rectal wall.
In the early stages of focal therapy, volume assessment was achieved by transperineal template mapping biopsy (TPM) which was invasive and had related adverse events [1]. There was therefore a need for a simple, non-invasive and accurate method to assess tumour volume. In other solid-organ cancers, imaging is routinely used to assess volume prior to any organ-sparing surgery such as partial nephrectomy or partial mastectomy. The aim of this article is to review the role of tumour volume assessment for focal therapy planning and to critically evaluate the most recent published evidence for different imaging modalities to estimate tumour volume.
Rationale for Tumour Volume Assessment
Focal Therapy Treatment Planning
Focal therapy encompasses a wide range of approaches that allow selective ablation of target areas. This may be delivered by a variety of energy modalities including high-intensity focused ultrasound (HIFU), cryotherapy, photodynamic therapy, focal laser ablation, focal brachytherapy, irreversible electroporation and radiofrequency ablation as well as interstitial drug injections. The principles of focal therapy planning are similar across all these techniques and rely on a balance between:
-
1.
Ensuring maximal safe energy delivery to area(s) of cancer with an appropriate margin
-
2.
Minimising damage to normal prostatic tissue and adjacent anatomical structures.
This requires precise mapping and contouring of the treatment area dependent on the volume of the lesion. An underestimation of tumour volume may result in inadequate coverage of the target area leaving residual significant disease and poor long-term efficacy. An overestimation of tumour volume increases the risk of damage to normal prostate tissue and structures such as neurovascular bundles, bladder neck, external sphincter and rectum. The coverage area of focal treatments maybe be lesion-based, quadrant, hemi-ablation or sub-total. The degree to which the precision of tumour volume is important will depend on the treatment strategy chosen as well as the energy source with newer devices offering greater precision of tissue destruction.
Risk Stratification: Clinically Insignificant Disease
The evidence for which men will benefit from active treatment of prostate cancer is evolving. It has been argued that low-grade and low-volume lesions do not have the typical hallmarks of cancer, certainly do not behave aggressively and may be regarded as clinically insignificant [2]. The ProtecT trial, which randomised men to active monitoring, surgery or radiotherapy in men diagnosed via PSA screening found no difference in prostate cancer-specific mortality at a median follow-up of 10 years [3]. The PIVOT and SPCG-4 RCTs show that the benefit of treatment resided in the high-risk group and possibly in intermediate-risk men too [4, 5]. There is a clear need for improved methods of risk stratifying men so that treatment can be directed towards those who are more likely to derive a cancer-specific mortality benefit.
The most widely used definition of clinically insignificant disease is based on the histopathological parameters set out by Stamey and Epstein [6]. Insignificant prostate cancer is defined on whole-mount prostatectomy as a tumour volume ranging from 0.2 to 0.5 cm3, no Gleason patterns 4 or 5 and organ confined. The original paper by Stamey et al. [6] described a single parameter of tumour volume ≥ 0.5 cm3 from a cystoprostatectomy series based on an 8% lifetime risk of being diagnosed with clinically significant cancer. Epstein et al. reported a volume threshold < 0.2 cm3 as being insignificant if the criteria of no capsular penetration were applied.
This tumour volume criterion has been generally considered too stringent and the definition of < 0.5 cm3 has been applied as the threshold for insignificant disease. In recent years, there has been a growing consensus that the 0.5 cm3 volume threshold remains too conservative. In a contemporary cystoprostectomy cohort applying the Stamey criteria, Winkler et al. [7] identified a higher threshold of 1.09 cm3. An analysis of the radical prostatectomy specimens from the European Randomized Study of Screening for Prostate Cancer found that grade and stage were the strongest determinants of lifetime risk estimates for prostate cancer [8]. When tumour volume was considered with organ confined Gleason 6 disease, a higher threshold of at least 1.3 ml for the index lesion and 2.5 ml for the total cancer volume was observed.
Patient Selection
Tumour volume has a fundamental role in patient selection for focal therapy and the success of treatment depends on identifying men with the appropriate burden of disease. The ideal case for focal therapy is a small-volume intermediate grade prostate cancer which is localised on multi-parametric MRI (mp-MRI) [9]. A recent expert consensus panel explored the range of tumour volumes which would be acceptable for focal therapy. The consensus was reached that an index lesion with an mp-MRI-derived volume up to 1.5 ml was suitable for treatment. There was further agreement that this volume threshold could be increased to 3 ml provided the lesion was localised to one hemi-gland and the energy source was capable of ablating this volume with an acceptable margin.
There has been a shift in the consensus opinion towards focal therapy as a strategy for treatment of intermediate rather than low-risk disease. The expert panel confirmed that Gleason 3+4 disease represents the optimum grade for focal therapy although there was a lack a consensus for treatment of higher risk disease. This shift towards treatment of intermediate-risk disease is in line with widespread consensus that men who are low risk should undergo active surveillance and even focal therapy would be overtreatment in this group. Furthermore, the promising medium term outcomes of focal therapy which are emerging from prospective cohort registry studies also support this trend. The 5-year outcomes of HIFU have been reported from a UK registry analysis of 625 patients showing failure-free survival was 88% with a median follow-up of 56 months [10]. The functional outcomes have been summarised in recent meta-analysis comparing patient reported outcomes measures of different whole gland therapies with HIFU and active surveillance [11]. The follow-up was inevitably shorter in the HIFU trials but there was no significant deterioration of sexual function or incontinence at 1 year. This is consistent with the findings of Yap et al. which found that although potency deteriorated at 1 and 3 months post-HIFU, it returned to baseline by 6 months [12].
The Need for an Alternative Volume Estimate
Prior to the emergence of focal therapy, there was a lack of an accurate and robust method of measuring pre-operative tumour volume. Previous attempts to determine tumour volume based on PSA level or digital rectal examination found that neither reliably correlated with volume on radical prostatectomy [13, 14]. The clinical Epstein criteria include indirect measurements of volume by number of positive cores and percentage of cancer on TRUS biopsy. Due to the sampling error inherent with this biopsy approach, these parameters have not been consistently shown to correlate with tumour volume on radical prostatectomy and have been calibrated to ensure significant disease is not missed rather than calibrated to prevent overtreatment [15,16,17,18].
Transperineal template mapping biopsy (TPM) has been shown to provide an accurate pre-operative risk assessment based on tumour volume definitions of clinically significant disease [19]. A cancer core length of ≥ 6 mm has been shown to predict lesions ≥ 0.5 ml in volume through a process of simulation against a radical prostatectomy cohort [19]. During the early stages of focal therapy, TPM was seen as an essential tool in selecting and risk stratifying men prior to treatment [20]. However, the high sampling density and requirement for a general anaesthetic placed a significant burden on both the patient and healthcare system [1].
The current biopsy pathway is being re-defined with a shift towards MRI-targeted biopsy. 3D histopathological models reconstructed from TPM biopsies were used to show that a single biopsy needle targeted to the maximum lesion diameter on mp-MRI leads to the correct Gleason grade in nearly all cases [21]. There is evidence from recent randomised controlled trials which confirm that a pre-biopsy MRI with or without targeted biopsy is superior to standard TRUS biopsy [22]. Although MRI-US-targeted biopsy may enhanced detection of clinically significant disease, the effect on estimation of tumour volume remains under investigation.
The studies attempting to re-calibrate maximum core length on targeted biopsy and tumour volume on radical prostatectomy have reported variable results [23, 24]. Baco et al. [23] found a weak correlation between maximum core length on elastic MR-TRUS image fusion and tumour volume on radical prostatectomy (r = 0.466). In this study, mp-MRI was a more accurate predictor of tumour volume (r = 0.663) than maximum cancer core length on targeted biopsy. Both cognitive and software-based targeted biopsy have a known targeting error which may be due to registration error [25], patient movement, prostate deformation or needle placement errors [26]. In comparison with TPM, this variability places an increasing importance on imaging to accurately detect and estimate tumour volume prior to focal therapy.
Multi-parametric MRI
The development of focal therapy has been facilitated by advances in imaging technologies. mp-MRI has become the standard imaging modality to detect and localise the index lesion. It provides an evaluation of the whole prostate in contrast to biopsy which samples only a very small proportion of the gland. There is extensive evidence that mp-MRI can reliably identify clinically significant disease ≥ 0.5 cm3 in volume with a high sensitivity and negative predictive value [27].
However, a volume measurement requires a higher level of spatial recognition than localisation or detection. There are an increasing number of studies evaluating volumetric assessment of lesions against radical prostatectomy specimens as the reference standard. Table 1 provides a summary of the studies including the differences in MRI techniques, contouring procedures and registration methods. There is significant heterogeneity across the literature with both underestimation and overestimation for tumour volume reported. There is also a high standard deviation within studies where tumour volume errors can range from − 136 to + 178%.
Evidence for Overestimation
There has been a rapid progression in technology and experience with mp-MRI over the last decade. The earlier studies were more likely to conclude that mp-MRI overestimated tumour volume but did not include software-based registration and relied on lower magnetic field strengths, including 0.5 T [42], non-multi-parametric sequences [30, 42]. Overestimation may also be attributable to methodological differences in sectioning the prostate gland and applying a correction factor for tissue shrinkage which varied from 1.14 to 1.5 [43, 44].
The decision regarding the degree of correction factor has a significant impact on the balance between overestimation and underestimation. Turkbey et al. [31] reported an identical analysis with and without a shrinkage factor correction. Without a shrinkage factor correction, mp-MRI overestimated tumour volume by 7% but after shrinkage factor correction, there was an underestimation of 7%. There is evidence that tissue shrinkage is not uniform between specimens and varies depending on fixation and mounting methods [45]. Recent studies have developed novel elastic 3D co-registration software to limit this bias and allow an individualised correction factor for each specimen [38].
Evidence for Underestimation
The studies conducted in the modern mp-MRI era generally describe that mp-MRI underestimates tumour volume, although there remains wide variability in the degree which ranges from 4 to 97% [40]. This underestimation occurs irrespective of MRI suspicion score or tumour characteristics[41] but may be biased by further challenges associated with preparation of the radical prostatectomy specimen.
Preparation of the histopathological slides leads to differences in angles, shape and depth compared with the MRI images. The sectioning plane of prostatectomy specimens may not reflect the MRI plane and the prostate shape can be altered by histopathological tissue processing. To improve the accuracy of registration, studies have used 3D patient-specific molds which improve the alignment of the specimens to the MRI images [41, 46]. However, these to not resolve all the registration challenges as while MRI slice thickness has improved to 1.5 mm, the majority of radical prostatectomy specimens underwent 3- to 5-mm step-sectioning (Table 1). The lack of a 1:1 slice correlation creates a systematic bias in the apex to base axis although the direction of effect depends on the exact method for calculating volume which is not reported in most studies. A study which stratified the level of underestimation by their axis reported that mp-MRI volumes were least accurate in the apex-base plane and most accurate in the axial plane with a MRI to histology slice ratio of 1.5:4 mm [41].
Histologically, it is speculated that underestimation may be attributable to the characteristic histological features at the boundary of the index lesion. Langer et al. have previously described histologically sparse areas of prostate cancer containing normal tissue intermixed with malignant epithelium which may not be visible on both T2WI and ADC maps [47]. These sparse regions may occur at the periphery of the index lesion meaning that mp-MRI will inherently underestimate the boundaries of visible lesions.
There is contradictory data on the effect of the MRI suspicion score, Gleason pattern and tumour volume on the degree of underestimation. The largest current study by Bratan et al. prospectively evaluated 202 radical prostatectomies and analysed the MRI and tumour characteristics which enhanced accuracy of mp-MRI-derived volume. The multi-variate analysis showed that Likert scores 4–5, Gleason score ≥ 7 and volume ≥2 ml had a more accurate mp-MRI volume estimation. Given that these large high-grade cancers are also easier to detect on mp-MRI [48], it might be expected that this would translate into a more accurate volume estimation.
However, using validated 3D co-registration software, Le Nobin et al. have reported the unexpected finding that larger tumours (> 1 ml) with a higher MRI or Gleason score had a more pronounced volume underestimation [38]. The authors suggest that this may be related to the more solid histological components of high-grade cancers meaning they manifest as clear dark areas on the ADC map. Given that PI-RADS v2 (Prostate Imaging-Reporting and Data System) recommends peripheral zone lesions are measured on ADC, it is possible that the radiologist’s attention is directed towards the darker areas which inherently excludes the less conspicuous surrounding non-solid lower-grade regions for the volume estimation.
Optimising MRI Volume Measurements
Optimal Method of Measurement
The PI-RADS v2.1 (Prostate Imaging-Reporting and Data System) guidelines provide the minimal requirements for measurement of volume which are to report a single measurement of a suspicious lesion on an axial image unless it is not clearly delineated, in which case the measurement should be on the image which best depicts the finding [49]. If the largest dimension of the lesion is on sagittal or coronal images, this measurement should also be reported.
The maximal diameter is a simple measurement which is feasible to obtain in clinical practice. It has been used as an inexpensive surrogate for tumour volume in radical prostatectomy specimens following studies comparing different methods for tumour size estimation [50]. The role of maximum diameter for mp-MRI-derived volume estimation is less well-established. Nakashima et al. [28] found that the maximal tumour diameters on MRI and radical prostatectomy specimens should be limited to tumours larger than 1.0 cm in diameter.
Alternative methods include three-dimensional quantification based on an ellipsoid formula or plainmetry. Planimetric volume measurement is presumed to be the most accurate technique and the majority of studies evaluating mp-MRI-derived tumour volumes adopt this approach. Plainmetry requires contouring of the lesion on each axial slice and places a significant additional time burden on the reporting radiologist.
At present, there remains no agreed method of measuring tumour volume on mp-MRI. Plainmetry is not routinely used in clinical practice although there is potential for this to change with further research into semi-automated or fully automated volumetric measurement software [51]. This issue was discussed in a recent expert consensus meeting and the panel concluded that there was not sufficient evidence to recommend any optimal method for measuring tumour volume on mp-MRI [52].
Optimal mp-MRI Sequence
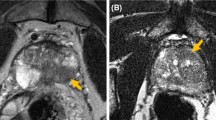
The early methods of estimating tumour volume were based on unenhanced T2W imaging alone. The addition of functional MRI sequences, such as diffusion-weighted imaging (DWI) and dynamic contrast-enhanced (DCE), improved the sensitivity and specificity for detection of clinically significant disease [48]. The effect on tumour volume assessment has been investigated in a limited number of studies [29, 32, 35] which find that T2W alone has a poor correlation with pathological volume (r = 0.21) [35]. DWI and ADC maps are consistently reported as the most accurate sequences with a high correlation coefficient (r = 0.75) [32]. Figure 1 shows an mp-MRI with an accurate tumour volume estimated on ADC map. DCE was the lowest performing sequence which is likely secondary to the lower spatial resolution of this sequence [29, 35].
An alternative approach is a measurement based on a combination of sequences acknowledging that all sequences underestimate tumour volume. Two studies have concluded that more accurate volume can be determined by the largest volume from any individual sequence [35, 45]. However, this approach leads to an overestimation of tumour volume by 16% [35]. Overall, the current evidence supports the use of DWI and ADC maps as the most accurate sequence for assessing tumour volume in the peripheral zone. These findings are reflected in PIRADS v2.1 which recommends that ADC should be used for assessing tumour volume in the peripheral zone [49]. T2W is recommended for the transition zone reflecting a lack of evidence for an optimal sequence for lesions in this region.
A potential benefit of ADC maps is that they allow a more objective assessment of volume as the values are proportional to the diffusion and perfusion characteristics of the tissue. There has been significant interest in establishing an objective ADC threshold to provide an automated method of assessing tumour volume. A voxelwise analysis by Mazaheri et al. [29] found that ADC cutoff values of 0.0014 and 0.0016 mm2/s improved the accuracy of volume measurements. The challenge for these models is that there is a large spread of ADC values inside the MRI lesion which can mean parts may not be automatically detected or there could be overdiagnosis depending on the threshold [53].
Other Factors
The zonal anatomical location of the index lesion could have an impact on accuracy of volume estimation. There are a few studies which categorise peripheral and transition zone lesions [36, 38, 41]. These suggest that transition zone lesions may be associated with more variability in tumour volume estimation [36] although peripheral zone lesions account for the majority in the analysis. The heterogeneous appearance of the transition zone makes it more challenging to define accurate boundaries in comparison with the peripheral zone lesions.
There are a wide range of other MRI technical characteristics which have been investigated in the existing literature which seem to have a limited effect on volume assessment. The influence of field strength was tested in a multi-variate analysis by Bratan et al. [37]. Despite the higher spatial resolution of 3 T, there was no significant difference in accuracy between 1.5 and 3 T. The impact of an endorectal coil has also been debated as it deforms the peripheral zone and may modify tumour contours in this region. Studies have been completed at 3 T and 1.5 T both with and without an endorectal coil. There is minimal evidence that the presence of an endorectal coil has a significant impact on tumour volume accuracy.
Optimising Focal Therapy Treatment Margins
To compensate for the underestimation of mp-MRI tumour volumes, there have been attempts to estimate an appropriate treatment margin to ensure full coverage of the index lesion. The 2015 Focal Therapy Consensus meeting recommended a circumferential margin of 5 mm around a lesion accounting for a 2–3 mm known registration error [25] and the underestimation by mp-MRI [54].
The principle of an adequate surgical margin surrounding a tumour is standard for all organ-conserving surgery. There has been extensive research into the appropriate margins for operations such as partial nephrectomy, partial penectomy and partial ureterectomy. For focal therapy, there are challenges to overcome due to the absence of a post-operative specimen to evaluate margin status. This has presented challenges for research into margin status and studies rely on extrapolation from radical prostatectomy specimens which have an inherent selection bias as well as the problems of accurate co-registration between histology and MRI.
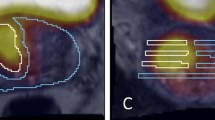
Cornud et al. [35] recommended a ‘target volume’ calculated on the largest tumour area on each axial slice from any sequence, but this resulted in an overestimation of pathological tumour volume by 44%. Recent work has attempted to quantify this into an exact margin using a simulated cylindrical treatment volume or the widest margin to achieve complete histological tumour distribution in all patients. These different methodologies have produced variable results with margins ranging from 5 mm [33] up to 13.5 mm [41, 55].
This variation highlights the need for individualised treatment margins which are determined based on the appropriate therapeutic risk-benefit ratio for each patient. The optimal margin is influenced by multiple patient-specific and operative factors. Tumour volume is an important component for the surgeon to consider along with other interrelated variables such as index lesion location, histological characteristics and energy modality.
Other Imaging Modalities
Transrectal Ultrasound
Transrectal ultrasound (TRUS) was the first major development in prostate cancer imaging. It revolutionised prostate cancer diagnostics by allowing the boundaries of the prostate to be visualised and providing the foundation for systematic biopsy [56]. Compared with other imaging modalities, TRUS is a fast, cost-effective and portable procedure which provides good soft-tissue contrast without the need for ionising radiation or administration of contrast agents. However, b-mode TRUS has demonstrated limited sensitivity and specificity for detecting and localising prostate cancer [57]. Given the lack of diagnostic accuracy, it is predictable that studies using b-mode TRUS have shown it is a poor predictor of tumour volume[13, 14].
Ultrasound is undergoing rapid technological advancements. Developments in high-resolution ultrasound operating up to 29 Hz allow superior spatial resolution over traditional b-mode imaging [58]. This may allow improved diagnostic accuracy and tumour volume assessment in the peripheral zone although the reduced penetration depth may limit assessment of the anterior gland. This has been combined with 3D scanning techniques to monitor the longitudinal growth of tumour volume in an orthotopic mouse model [59]. The 3D rendering showed good correlation for prostate tumour volume measurements performed in vivo with autopsy (r = 0.95).
Similar to the development of mp-MRI, there has been interest in combining US modalities into a ‘multi-parametric ultrasound’. If a combination of anatomical and functional parameters can be shown to accurately detect and localise the index lesion, this will be a significant step forward for focal therapy treatment planning due to the real-time monitoring available through ultrasound. The early results for multi-parametric ultrasound are encouraging [60] and there are ongoing randomised controlled trials which will provide a robust comparison with mp-MRI [61]. At present, there is limited data on the performance of these modalities for determining tumour volume while we await further evidence on diagnostic accuracy.
CT
Although computed tomography (CT) is a widely used modality for the detection and localisation in many malignancies, it has a limited role in prostate cancer localisation or focal therapy planning and due to the inherent lack of soft tissue, contrast resolution so cannot reliably visualise prostate zonal anatomy or estimate volume of tumours. The prostate will generally appear on an unenhanced CT as a homogenous soft tissue structure, and prostate cancer will not be visualised unless gross extension is present [62].
PET/CT
Although the role of choline PET/CT as a staging investigation is well established, the spatial resolution is limited to around 5 mm so its ability to accurately localise prostate cancer and estimate tumour volume has been debated. The role of choline PET/CT for focal therapy planning has not been investigated but it has been evaluated for delineation of the dominant intra-prostatic lesion for intensity modulated radiation therapy (IMRT) focal dose escalation. This requires a similar process of contouring the lesion as focal therapy planning and at present, mp-MRI is the standard technique.
The results for choline-based markers suggest they have a limited role for evaluation of tumour volume. Multiple studies have shown a poor correlation with tumour volume (r = 0.3) [63] and Bundschuh et al. found that the choline uptake pattern was inconsistent and no suitable threshold could be identified to fit histological volume [64]. Instead PSMA ligands such as 68Ga-labeled HBED-CC-PSMA or 18F-labeled DCFPyl are emerging as a promising alternative and appear to be more sensitive for detection of local and metastatic disease. The majority of studies focus on the detection of recurrent or metastatic disease. Preliminary studies evaluating PSMA/PET for IMRT focal dose have found that PET-derived volumes are significantly larger in some patients compared with MRI or prostatectomy volumes [65, 66] and that further correlation studies with co-registration of histopathological data are required.
Conclusion
An accurate estimation of tumour volume is essential for focal therapy treatment planning. If the tumour volume is overestimated, the risk of complications increases, while an underestimation reduces the chance of effective cancer control. The advances in mp-MRI have provided a non-invasive method of assessing tumour volume although this may be underestimated by all sequences. At present, there are no other viable image-based alternatives for assessing tumour volume.
There is considerable variability across the literature in the results for all studies which likely reflects the variability in imaging techniques and methods of co-registration between studies. For further research to progress in this area, there needs to be a robust method for co-registration of histopathological data with imaging.
References
Miah S, Eldred-Evans D, Simmons LA, Shah TT, Kanthabalan A, Arya M, et al. Patient reported outcome measures for transperineal template prostate mapping biopsies in the PICTURE study. J Urol. 2018;200(6):1235–40.
Ahmed HU, Arya M, Freeman A, Emberton M. Do low-grade and low-volume prostate cancers bear the hallmarks of malignancy? Lancet Oncol. 2012;13(11):e509–e17.
Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415–24.
Bill-Axelson A, Holmberg L, Garmo H, Rider JR, Taari K, Busch C, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2014;370(10):932–42. https://doi.org/10.1056/NEJMoa1311593.
Wilt TJ, Jones KM, Barry MJ, Andriole GL, Culkin D, Wheeler T, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med. 2017;377(2):132–42.
Stamey TA, Freiha FS, McNeal JE, Redwine EA, Whittemore AS, Schmid HP. Localized prostate cancer. Relationship of tumor volume to clinical significance for treatment of prostate cancer. Cancer. 1993;71(S3):933–8.
Winkler MH, Livni N, Mannion EM, Hrouda D, Christmas T. Characteristics of incidental prostatic adenocarcinoma in contemporary radical cystoprostatectomy specimens. BJU Int. 2007;99(3):554–8.
Wolters T, Roobol MJ, van Leeuwen PJ, van den Bergh RCN, Hoedemaeker RF, van Leenders GJLH, et al. A critical analysis of the tumor volume threshold for clinically insignificant prostate cancer using a data set of a randomized screening trial. J Urol. 2011;185(1):121–5. https://doi.org/10.1016/j.juro.2010.08.082.
Rivas JG, Emberton M. Tissue preservation: active surveillance and focal therapy as complimentary strategies. In: Klotz L, editor. Active surveillance for localized prostate cancer: a new paradigm for clinical management. Cham: Springer International Publishing; 2018. p. 217–27.
Guillaumier S, Peters M, Arya M, Afzal N, Charman S, Dudderidge T, et al. A multicentre study of 5-year outcomes following focal therapy in treating clinically significant nonmetastatic prostate cancer. Eur Urol. 2018. https://doi.org/10.1016/j.eururo.2018.06.006.
Ávila M, Patel L, López S, Cortés-Sanabria L, Garin O, Pont À, et al. Patient-reported outcomes after treatment for clinically localized prostate cancer: a systematic review and meta-analysis. Cancer Treat Rev. 2018;66:23–44. https://doi.org/10.1016/j.ctrv.2018.03.005.
Guillaumier S, Peters M, Arya M, Afzal N, Charman S, Dudderidge T, et al. A multicentre study of 5-year outcomes following focal therapy in treating clinically significant nonmetastatic prostate cancer. Eur Urol. 2018;74(4):422–9. https://doi.org/10.1016/j.eururo.2018.06.006.
Palken M, Cobb OE, Warren BH, Hoak DC. Prostate cancer: correlation of digital rectal examination, transrectal ultrasound and prostate specific antigen levels with tumor volumes in radical prostatectomy specimens. J Urol. 1990;143(6):1155–62.
McSherry SA, Levy F, Schiebler ML, Keefe B, Dent GA, Mohler JL. Preoperative prediction of pathological tumor volume and stage in clinically localized prostate cancer: comparison of digital rectal examination, transrectal ultrasonography and magnetic resonance imaging. J Urol. 1991;146(1):85–9. https://doi.org/10.1016/S0022-5347(17)37720-0.
Olumi AF, Richie JP, Schultz DJ, D’Amico AV. Calculated volume of prostate cancer identifies patients with clinical stage T1C disease at high risk of biochemical recurrence after radical prostatectomy: a preliminary study. Urology. 2000;56(2):273–7.
Freedland SJ, Aronson WJ, Terris MK, Kane CJ, Amling CL, Dorey F, et al. Percent of prostate needle biopsy cores with cancer is significant independent predictor of prostate specific antigen recurrence following radical prostatectomy: results from SEARCH database. J Urol. 2003;169(6):2136–41. https://doi.org/10.1097/01.ju.0000065588.82511.06.
Cupp MR, Bostwick DG, Myers RP, Oesterling JE. The volume of prostate cancer in the biopsy specimen cannot reliably predict the quantity of cancer in the radical prostatectomy specimen on an individual basis. J Urol. 1995;153(5):1543–8. https://doi.org/10.1016/S0022-5347(01)67458-5.
Dietrick DD, McNeal JE, Stamey TA. Core cancer length in ultrasound-guided systematic sextant biopsies: a preoperative evaluation of prostate cancer volume. Urology. 1995;45(6):987–92. https://doi.org/10.1016/S0090-4295(99)80119-8.
Ahmed HU, Hu Y, Carter T, Arumainayagam N, Lecornet E, Freeman A, et al. Characterizing clinically significant prostate cancer using template prostate mapping biopsy. J Urol. 2011;186(2):458–64. https://doi.org/10.1016/j.juro.2011.03.147.
Onik G, Barzell W, editors. Transperineal 3D mapping biopsy of the prostate: an essential tool in selecting patients for focal prostate cancer therapy. Urol Oncol: Seminars and Original Investigations; 2008: Elsevier.
Bosaily AE-S, Valerio M, Hu Y, Freeman A, Jameson C, Brown L, et al. The concordance between the volume hotspot and the grade hotspot: a 3-D reconstructive model using the pathology outputs from the PROMIS trial. Prostate Cancer Prostatic Dis. 2016;19(3):258–63.
Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;378(19):1767–77.
Baco E, Ukimura O, Rud E, Vlatkovic L, Svindland A, Aron M, et al. Magnetic resonance imaging–transectal ultrasound image-fusion biopsies accurately characterize the index tumor: correlation with step-sectioned radical prostatectomy specimens in 135 patients. Eur Urol. 2015;67(4):787–94. https://doi.org/10.1016/j.eururo.2014.08.077.
Matsugasumi T, Baco E, Palmer S, Aron M, Sato Y, Fukuda N, et al. Prostate cancer volume estimation by combining magnetic resonance imaging and targeted biopsy proven cancer core length: correlation with cancer volume. J Urol. 2015;194(4):957–65. https://doi.org/10.1016/j.juro.2015.04.075.
Hu Y, Ahmed HU, Taylor Z, Allen C, Emberton M, Hawkes D, et al. MR to ultrasound registration for image-guided prostate interventions. Med Image Anal. 2012;16(3):687–703. https://doi.org/10.1016/j.media.2010.11.003.
Cash H, Günzel K, Maxeiner A, Stephan C, Fischer T, Durmus T, et al. Prostate cancer detection on transrectal ultrasonography-guided random biopsy despite negative real-time magnetic resonance imaging/ultrasonography fusion-guided targeted biopsy: reasons for targeted biopsy failure. BJU Int. 2015;118(1):35–43. https://doi.org/10.1111/bju.13327.
Ahmed HU, Bosaily AE-S, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389(10071):815–22.
Nakashima J, Tanimoto A, Imai Y, Mukai M, Horiguchi Y, Nakagawa K, et al. Endorectal MRI for prediction of tumor site, tumor size, and local extension of prostate cancer. Urology. 2004;64(1):101–5. https://doi.org/10.1016/j.urology.2004.02.036.
Mazaheri Y, Hricak H, Fine SW, Akin O, Shukla-Dave A, Ishill NM, et al. Prostate tumor volume measurement with combined T2-weighted imaging and diffusion-weighted MR: correlation with pathologic tumor volume. Radiology. 2009;252(2):449–57. https://doi.org/10.1148/radiol.2523081423.
Lemaitre L, Puech P, Poncelet E, Bouye S, Leroy X, Biserte J, et al. Dynamic contrast-enhanced MRI of anterior prostate cancer: morphometric assessment and correlation with radical prostatectomy findings. Eur Radiol. 2009;19(2):470–80. https://doi.org/10.1007/s00330-008-1153-0.
Turkbey B, Mani H, Aras O, Rastinehad AR, Shah V, Bernardo M, et al. Correlation of magnetic resonance imaging tumor volume with histopathology. J Urol. 2012;188(4):1157–63. https://doi.org/10.1016/j.juro.2012.06.011.
Isebaert S, Van den Bergh L, Haustermans K, Joniau S, Lerut E, De Wever L, et al. Multiparametric MRI for prostate cancer localization in correlation to whole-mount histopathology. J Magn Reson Imaging. 2012;37(6):1392–401. https://doi.org/10.1002/jmri.23938.
Anwar M, Westphalen AC, Jung AJ, Noworolski SM, Simko JP, Kurhanewicz J, et al. Role of endorectal MR imaging and MR spectroscopic imaging in defining treatable intraprostatic tumor foci in prostate cancer: quantitative analysis of imaging contour compared to whole-mount histopathology. Radiother Oncol. 2014;110(2):303–8. https://doi.org/10.1016/j.radonc.2013.12.003.
Engelhard K, Labanaris AP, Bogner K, Lubke L, Dworak O, Kuhn R. How good is post-biopsy multiparametric magnetic resonance imaging in detecting and characterising the index lesion of localised prostate cancer? Scand J Urol. 2014;48(6):499–505. https://doi.org/10.3109/21681805.2014.907338.
Cornud F, Khoury G, Bouazza N, Beuvon F, Peyromaure M, Flam T, et al. Tumor target volume for focal therapy of prostate cancer-does multiparametric magnetic resonance imaging allow for a reliable estimation? J Urol. 2014;191(5):1272–9. https://doi.org/10.1016/j.juro.2013.12.006.
Rud E, Klotz D, Rennesund K, Baco E, Berge V, Lien D, et al. Detection of the index tumour and tumour volume in prostate cancer using T2-weighted and diffusion-weighted magnetic resonance imaging (MRI) alone. BJU Int. 2014;114(6b):E32–42. https://doi.org/10.1111/bju.12637.
Bratan F, Melodelima C, Souchon R, Hoang Dinh A, Mege-Lechevallier F, Crouzet S, et al. How accurate is multiparametric MR imaging in evaluation of prostate cancer volume? Radiology. 2015;275(1):144–54. https://doi.org/10.1148/radiol.14140524.
Le Nobin J, Orczyk C, Deng F-M, Melamed J, Rusinek H, Taneja SS, et al. Prostate tumour volumes: evaluation of the agreement between magnetic resonance imaging and histology using novel co-registration software. BJU Int. 2014;114(0):E105–E12. https://doi.org/10.1111/bju.12750.
Radtke JP, Schwab C, Wolf MB, Freitag MT, Alt CD, Kesch C, et al. Multiparametric magnetic resonance imaging (MRI) and MRI–transrectal ultrasound fusion biopsy for index tumor detection: correlation with radical prostatectomy specimen. Eur Urol. 2016;70(5):846–53. https://doi.org/10.1016/j.eururo.2015.12.052.
Martorana E, Pirola Giacomo M, Scialpi M, Micali S, Iseppi A, Bonetti Luca R, et al. Lesion volume predicts prostate cancer risk and aggressiveness: validation of its value alone and matched with prostate imaging reporting and data system score. BJU Int. 2016;120(1):92–103. https://doi.org/10.1111/bju.13649.
Priester A, Natarajan S, Khoshnoodi P, Margolis DJ, Raman SS, Reiter RE, et al. Magnetic resonance imaging underestimation of prostate cancer geometry: use of patient specific molds to correlate images with whole mount pathology. J Urol. 2017;197(2):320–6. https://doi.org/10.1016/j.juro.2016.07.084.
Lencioni R, Menchi I, Paolicchi A, Carini M, Amorosi A, Bartolozzi C. Prediction of pathological tumor volume in clinically localized prostate cancer: value of endorectal coil magnetic resonance imaging. Magma (New York, NY). 1997;5(2):117–21.
Stamey TA, McNeal JE, Freiha FS, Redwine E. Morphometric and clinical studies on 68 consecutive radical prostatectomies. J Urol. 1988;139(6):1235–41.
Schned AR, Wheeler KJ, Hodorowski CA, Heaney JA, Ernstoff MS, Amdur RJ, et al. Tissue-shrinkage correction factor in the calculation of prostate cancer volume. Am J Surg Pathol. 1996;20(12):1501–6.
Bratan F, Niaf E, Melodelima C, Chesnais AL, Souchon R, Mège-Lechevallier F, et al. Influence of imaging and histological factors on prostate cancer detection and localisation on multiparametric MRI: a prospective study. Eur Radiol. 2013;23(7):2019–29. https://doi.org/10.1007/s00330-013-2795-0.
Partanen A, Yerram NK, Trivedi H, Dreher MR, Oila J, Hoang AN, et al. Magnetic resonance imaging (MRI)-guided transurethral ultrasound therapy of the prostate: a preclinical study with radiological and pathological correlation using customised MRI-based moulds. BJU Int. 2013;112(4):508–16. https://doi.org/10.1111/bju.12126.
Langer DL, van der Kwast TH, Evans AJ, Sun L, Yaffe MJ, Trachtenberg J, et al. Intermixed normal tissue within prostate cancer: effect on MR imaging measurements of apparent diffusion coefficient and T2--sparse versus dense cancers. Radiology. 2008;249(3):900–8. https://doi.org/10.1148/radiol.2493080236.
Fütterer JJ, Briganti A, De Visschere P, Emberton M, Giannarini G, Kirkham A, et al. Can clinically significant prostate cancer be detected with multiparametric magnetic resonance imaging? A systematic review of the literature. Eur Urol. 2015;68(6):1045–53. https://doi.org/10.1016/j.eururo.2015.01.013.
Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, et al. PI-RADS prostate imaging–reporting and data system: 2015, version 2. Eur Urol. 2016;69(1):16–40.
Renshaw AA, Chang H, D’Amico AV. Estimation of tumor volume in radical prostatectomy specimens in routine clinical practice. Am J Clin Pathol. 1997;107(6):704–8.
Marin L, Ezziane M, Comperat E, Mozer P, Cancel-Tassin G, Coté JF, et al. Comparison of semi-automated and manual methods to measure the volume of prostate cancer on magnetic resonance imaging. Diagn Interv Imaging. 2017;98(5):423–8. https://doi.org/10.1016/j.diii.2017.02.004.
Moore CM, Giganti F, Albertsen P, Allen C, Bangma C, Briganti A, et al. Reporting magnetic resonance imaging in men on active surveillance for prostate cancer: the PRECISE recommendations—a report of a European School of Oncology Task Force. Eur Urol. 2017;71(4):648–55. https://doi.org/10.1016/j.eururo.2016.06.011.
Borren A, Groenendaal G, Moman MR, Boeken Kruger AE, van Diest PJ, van Vulpen M, et al. Accurate prostate tumour detection with multiparametric magnetic resonance imaging: dependence on histological properties. Acta Oncol. 2014;53(1):88–95. https://doi.org/10.3109/0284186X.2013.837581.
Donaldson IA, Alonzi R, Barratt D, Barret E, Berge V, Bott S, et al. Focal therapy: patients, interventions, and outcomes—a report from a consensus meeting. Eur Urol. 2015;67(4):771–7. https://doi.org/10.1016/j.eururo.2014.09.018.
Le Nobin J, Rosenkrantz AB, Villers A, Orczyk C, Deng F-M, Melamed J, et al. Image guided focal therapy of magnetic resonance imaging visible prostate cancer: defining a 3-dimensional treatment margin based on magnetic resonance imaging-histology co-registration analysis. J Urol. 2015;194(2):364–70. https://doi.org/10.1016/j.juro.2015.02.080.
Lee F, Torp-Pedersen S, Siders D, Littrup P, McLeary R. Transrectal ultrasound in the diagnosis and staging of prostatic carcinoma. Radiology. 1989;170(3):609–15.
Carter HB, Hamper UM, Sheth S, Sanders RC, Epstein JI, Walsh PC. Evaluation of transrectal ultrasound in the early detection of prostate cancer. J Urol. 1989;142(4):1008–10.
Ghai S, Eure G, Fradet V, Hyndman ME, McGrath T, Wodlinger B, et al. Assessing cancer risk on novel 29 MHz micro-ultrasound images of the prostate: creation of the micro-ultrasound protocol for prostate risk identification. J Urol. 2016;196(2):562–9.
Ni J, Cozzi P, Hung T-T, Hao J, Graham P, Li Y. Monitoring prostate tumor growth in an orthotopic mouse model using three-dimensional ultrasound imaging technique. Transl Oncol. 2016;9(1):41–5. https://doi.org/10.1016/j.tranon.2015.11.011.
Postema A, Mischi M, de la Rosette J, Wijkstra H. Multiparametric ultrasound in the detection of prostate cancer: a systematic review. World J Urol. 2015;33(11):1651–9. https://doi.org/10.1007/s00345-015-1523-6.
Grey A, Scott R, Charman S, Van Der Meulen J, Frinking P, Acher P, et al. The CADMUS trial – multi-parametric ultrasound targeted biopsies compared to multi-parametric MRI targeted biopsies in the diagnosis of clinically significant prostate cancer. Contemp Clin Trials. 2018;66:86–92. https://doi.org/10.1016/j.cct.2017.10.011.
Turkbey B, Albert PS, Kurdziel K, Choyke PL. Imaging localized prostate cancer: current approaches and new developments. Am J Roentgenol. 2009;192(6):1471–80. https://doi.org/10.2214/AJR.09.2527.
Van den Bergh L, Koole M, Isebaert S, Joniau S, Deroose CM, Oyen R, et al. Is there an additional value of 11C-choline PET-CT to T2-weighted MRI images in the localization of intraprostatic tumor nodules? Int J Radiat Oncol Biol Physics. 2012;83(5):1486–92. https://doi.org/10.1016/j.ijrobp.2011.10.046.
Bundschuh RA, Wendl CM, Weirich G, Eiber M, Souvatzoglou M, Treiber U, et al. Tumour volume delineation in prostate cancer assessed by [11C]choline PET/CT: validation with surgical specimens. Eur J Nucl Med Mol Imaging. 2013;40(6):824–31. https://doi.org/10.1007/s00259-013-2345-7.
Rowe SP, Gage KL, Faraj SF, Macura KJ, Cornish TC, Gonzalez-Roibon N, et al. 18F-DCFBC PET/CT for PSMA-based detection and characterization of primary prostate cancer. J Nucl Med. 2015;56(7):1003–10. https://doi.org/10.2967/jnumed.115.154336.
Zamboglou C, Wieser G, Hennies S, Rempel I, Kirste S, Soschynski M, et al. MRI versus (6)(8)Ga-PSMA PET/CT for gross tumour volume delineation in radiation treatment planning of primary prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43(5):889–97. https://doi.org/10.1007/s00259-015-3257-5.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on New Imaging Techniques
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eldred-Evans, D., Tam, H., Smith, A.P.T. et al. Use of Imaging to Optimise Prostate Cancer Tumour Volume Assessment for Focal Therapy Planning. Curr Urol Rep 21, 38 (2020). https://doi.org/10.1007/s11934-020-00987-y
Published:
DOI: https://doi.org/10.1007/s11934-020-00987-y