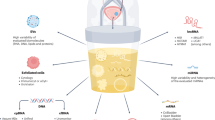
Abstract
Purpose of Review
We summarize the current literature regarding the available urinary biomarkers for the detection and surveillance of bladder cancer.
Recent Findings
Four urinary biomarkers have FDA approval for the detection of bladder cancer; however, they have not supplanted cystoscopy and urine cytology as the gold standard. Recent technological advances in next-generation sequencing have allowed the field of urinary biomarker research to move beyond protein biomarkers and now include genomic, transcriptomic, and epigenetic panels.
Summary
The search for a noninvasive, inexpensive urinary biomarker for the detection of bladder cancer that can replace cystoscopy and cytology continues. There are several promising genomic, transcriptomic, and epigenetic marker panels in development; however, these new tests require further prospective validation before widespread clinical implementation.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. https://doi.org/10.3322/caac.21442.
Tan WS, Feber A, Sarpong R, Khetrapal P, Rodney S, Jalil R, et al. Who should be investigated for haematuria? Results of a contemporary prospective observational study of 3556 patients. Eur Urol. 2018;74(1):10–4. https://doi.org/10.1016/j.eururo.2018.03.008.
Davis RJ JS, Barocas DA, Castle EP, Lang EK, Leveillee RJ, Messing EM, Miller SD, Peterson AC, Turk T, Weitzel W. Diagnosis, evaluation, and follow-up of asymptomatic microhematuria (AMH) in adults: AUA Guideline. 2012. http://www.auanet.org/guidelines/asymptomatic-microhematuria-(2012-reviewed-for-currency-2016). Accessed 8/7/2018.
Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63(2):234–41. https://doi.org/10.1016/j.eururo.2012.07.033.
Kamat AM, Hahn NM, Efstathiou JA, Lerner SP, Malmstrom PU, Choi W, et al. Bladder cancer. Lancet. 2016;388(10061):2796–810. https://doi.org/10.1016/S0140-6736(16)30512-8.
Cambier S, Sylvester RJ, Collette L, Gontero P, Brausi MA, van Andel G, et al. EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non–muscle-invasive stage ta–T1 urothelial bladder cancer patients treated with 1–3 years of maintenance Bacillus Calmette-Guérin. Eur Urol. 2016;69(1):60–9. https://doi.org/10.1016/j.eururo.2015.06.045.
Chang SS, Boorjian SA, Chou R, Clark PE, Daneshmand S, Konety BR, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J Urol. 2016;196(4):1021–9. https://doi.org/10.1016/j.juro.2016.06.049.
Burke DM, Shackley DC, O’Reilly PH. The community-based morbidity of flexible cystoscopy. BJU International. 2002;89(4):347–9. https://doi.org/10.1046/j.1464-4096.2001.01899.x.
Xylinas E, Kluth LA, Rieken M, Karakiewicz PI, Lotan Y, Shariat SF. Urine markers for detection and surveillance of bladder cancer. Urol Oncol. 2014;32(3):222–9. https://doi.org/10.1016/j.urolonc.2013.06.001.
Leiblich A. Recent developments in the search for urinary biomarkers in bladder cancer. Curr Urol Reports. 2017;18(12):100. https://doi.org/10.1007/s11934-017-0748-x.
Jocham D, Stepp H, Waidelich R. Photodynamic diagnosis in urology: state-of-the-art. Eur Urol. 2008;53(6):1138–48. https://doi.org/10.1016/j.eururo.2007.11.048.
Burchardt M, Burchardt T, Shabsigh A, De La Taille A, Benson MC, Sawczuk I. Current concepts in biomarker technology for bladder cancers. Clin Chem. 2000;46(5):595–605.
Gregoire M, Fradet Y, Meyer F, Tetu B, Bois R, Bedard G, et al. Diagnostic accuracy of urinary cytology, and deoxyribonucleic acid flow cytometry and cytology on bladder washings during followup for bladder tumors. J Urol. 1997;157(5):1660–4.
Lotan Y, Roehrborn CG. Sensitivity and specificity of commonly available bladder tumor markers versus cytology: results of a comprehensive literature review and meta-analyses. Urology. 2003;61(1):109–18 discussion 18.
Karakiewicz PI, Benayoun S, Zippe C, Ludecke G, Boman H, Sanchez-Carbayo M, et al. Institutional variability in the accuracy of urinary cytology for predicting recurrence of transitional cell carcinoma of the bladder. BJU Int. 2006;97(5):997–1001. https://doi.org/10.1111/j.1464-410X.2006.06036.x.
•• Lotan Y, O’Sullivan P, Raman JD, Shariat SF, Kavalieris L, Frampton C, et al. Clinical comparison of noninvasive urine tests for ruling out recurrent urothelial carcinoma. Urol Oncol. 2017;35(8):531 e15–22. https://doi.org/10.1016/j.urolonc.2017.03.008 Prospective study showing that Cxbladder Monitor, a transcriptomic biomarker assay, had significantly improved sensitivity and specificity compared to cytology and NMP22.
Daneshmand S, Patel S, Lotan Y, Pohar K, Trabulsi E, Woods M, et al. Efficacy and safety of blue light flexible cystoscopy with hexaminolevulinate in the surveillance of bladder cancer: a phase III, comparative, multicenter study. J Urol. 2018;199(5):1158–65. https://doi.org/10.1016/j.juro.2017.11.096.
Lokeshwar VB, Soloway MS. Current bladder tumor tests: does their projected utility fulfill clinical necessity? J Urol. 2001;165(4):1067–77.
Lokeshwar VB, Habuchi T, Grossman HB, Murphy WM, Hautmann SH, Hemstreet GP 3rd, et al. Bladder tumor markers beyond cytology: international consensus panel on bladder tumor markers. Urology. 2005;66(6 Suppl 1):35–63. https://doi.org/10.1016/j.urology.2005.08.064.
Sarosdy MF, Hudson MA, Ellis WJ, Soloway MS, deVere White R, Sheinfeld J et al. Improved detection of recurrent bladder cancer using the Bard BTA stat test. Urology 1997;50(3):349–353.
Kinders R, Jones T, Root R, Bruce C, Murchison H, Corey M, et al. Complement factor H or a related protein is a marker for transitional cell cancer of the bladder. Clin Cancer Res. 1998;4(10):2511–20.
Malkowicz SB. The application of human complement factor H-related protein (BTA TRAK) in monitoring patients with bladder cancer. Urol Clin North Am. 2000;27(1):63–73 ix.
Sharma S, Zippe CD, Pandrangi L, Nelson D, Agarwal A. Exclusion criteria enhance the specificity and positive predictive value of NMP22 and BTA stat. J Urol. 1999;162(1):53–7.
Boman H, Hedelin H, Holmang S. Four bladder tumor markers have a disappointingly low sensitivity for small size and low grade recurrence. J Urol. 2002;167(1):80–3.
Poulakis V, Witzsch U, De Vries R, Altmannsberger HM, Manyak MJ, Becht E. A comparison of urinary nuclear matrix protein-22 and bladder tumour antigen tests with voided urinary cytology in detecting and following bladder cancer: the prognostic value of false-positive results. BJU Int. 2001;88(7):692–701.
Wiener HG, Mian C, Haitel A, Pycha A, Schatzl G, Marberger M. Can urine bound diagnostic tests replace cystoscopy in the management of bladder cancer? J Urol. 1998;159(6):1876–80.
Pode D, Shapiro A, Wald M, Nativ O, Laufer M, Kaver I. Noninvasive detection of bladder cancer with the BTA stat test. J Urol. 1999;161(2):443–6.
Ramakumar S, Bhuiyan J, Besse JA, Roberts SG, Wollan PC, Blute ML, et al. Comparison of screening methods in the detection of bladder cancer. J Urol. 1999;161(2):388–94.
Nasuti JF, Gomella LG, Ismial M, Bibbo M. Utility of the BTA stat test kit for bladder cancer screening. Diagn Cytopathol. 1999;21(1):27–9.
Serretta V, Pomara G, Rizzo I, Esposito E. Urinary BTA-stat, BTA-trak and NMP22 in surveillance after TUR of recurrent superficial transitional cell carcinoma of the bladder. Eur Urol. 2000;38(4):419–25. https://doi.org/10.1159/000020318.
Giannopoulos A, Manousakas T, Mitropoulos D, Botsoli-Stergiou E, Constantinides C, Giannopoulou M, et al. Comparative evaluation of the BTAstat test, NMP22, and voided urine cytology in the detection of primary and recurrent bladder tumors. Urology. 2000;55(6):871–5.
Raitanen MP, Marttila T, Nurmi M, Ala-Opas M, Nieminen P, Aine R, et al. Human complement factor H related protein test for monitoring bladder cancer. J Urol. 2001;165(2):374–7. https://doi.org/10.1097/00005392-200102000-00005.
Babjuk M, Kostirova M, Mudra K, Pecher S, Smolova H, Pecen L, et al. Qualitative and quantitative detection of urinary human complement factor H-related protein (BTA stat and BTA TRAK) and fragments of cytokeratins 8, 18 (UBC rapid and UBC IRMA) as markers for transitional cell carcinoma of the bladder. Eur Urol. 2002;41(1):34–9.
Schroeder GL, Lorenzo-Gomez MF, Hautmann SH, Friedrich MG, Ekici S, Huland H, et al. A side by side comparison of cytology and biomarkers for bladder cancer detection. J Urol. 2004;172(3):1123–6. https://doi.org/10.1097/01.ju.0000134347.14643.ab.
Babjuk M, Soukup V, Pesl M, Kostirova M, Drncova E, Smolova H, et al. Urinary cytology and quantitative BTA and UBC tests in surveillance of patients with pTapT1 bladder urothelial carcinoma. Urology. 2008;71(4):718–22. https://doi.org/10.1016/j.urology.2007.12.021.
Ellis WJ, Blumenstein BA, Ishak LM, Enfield DL. Clinical evaluation of the BTA TRAK assay and comparison to voided urine cytology and the Bard BTA test in patients with recurrent bladder tumors. The Multi Center Study Group. Urology. 1997;50(6):882–887.
Glas AS, Roos D, Deutekom M, Zwinderman AH, Bossuyt PM, Kurth KH. Tumor markers in the diagnosis of primary bladder cancer. A systematic review. J Urol. 2003;169(6):1975–82. https://doi.org/10.1097/01.ju.0000067461.30468.6d.
van Rhijn BW, van der Poel HG, van der Kwast TH. Urine markers for bladder cancer surveillance: a systematic review. Eur Urol. 2005;47(6):736–48. https://doi.org/10.1016/j.eururo.2005.03.014.
Raitanen MP, FinnBladder G. The role of BTA stat test in follow-up of patients with bladder cancer: results from FinnBladder studies. World J Urol. 2008;26(1):45–50. https://doi.org/10.1007/s00345-007-0230-3.
Lokeshwar VB, Schroeder GL, Selzer MG, Hautmann SH, Posey JT, Duncan RC, et al. Bladder tumor markers for monitoring recurrence and screening comparison of hyaluronic acid-hyaluronidase and BTA-stat tests. Cancer. 2002;95(1):61–72. https://doi.org/10.1002/cncr.10652.
Landman J, Chang Y, Kavaler E, Droller MJ, Liu BC. Sensitivity and specificity of NMP-22, telomerase, and BTA in the detection of human bladder cancer. Urology. 1998;52(3):398–402.
Mbeutcha A, Lucca I, Mathieu R, Lotan Y, Shariat SF. Current status of urinary biomarkers for detection and surveillance of bladder Cancer. Urol Clin North Am. 2016;43(1):47–62. https://doi.org/10.1016/j.ucl.2015.08.005.
Hughes JH, Katz RL, Rodriguez-Villanueva J, Kidd L, Dinney C, Grossman HB, et al. Urinary nuclear matrix protein 22 (NMP22): a diagnostic adjunct to urine cytologic examination for the detection of recurrent transitional-cell carcinoma of the bladder. Diagn Cytopathol. 1999;20(5):285–90.
Soloway MS, Briggman V, Carpinito GA, Chodak GW, Church PA, Lamm DL, et al. Use of a new tumor marker, urinary NMP22, in the detection of occult or rapidly recurring transitional cell carcinoma of the urinary tract following surgical treatment. J Urol. 1996;156(2 Pt 1):363–7.
Shariat SF, Marberger MJ, Lotan Y, Sanchez-Carbayo M, Zippe C, Ludecke G, et al. Variability in the performance of nuclear matrix protein 22 for the detection of bladder cancer. J Urol. 2006;176(3):919–26; discussion 26. https://doi.org/10.1016/j.juro.2006.04.017.
Hutterer GC, Karakiewicz PI, Zippe C, Ludecke G, Boman H, Sanchez-Carbayo M, et al. Urinary cytology and nuclear matrix protein 22 in the detection of bladder cancer recurrence other than transitional cell carcinoma. BJU Int. 2008;101(5):561–5. https://doi.org/10.1111/j.1464-410X.2007.07352.x.
Barbieri CE, Cha EK, Chromecki TF, Dunning A, Lotan Y, Svatek RS, et al. Decision curve analysis assessing the clinical benefit of NMP22 in the detection of bladder cancer: secondary analysis of a prospective trial. BJU Int. 2012;109(5):685–90. https://doi.org/10.1111/j.1464-410X.2011.010419.x.
Shariat SF, Savage C, Chromecki TF, Sun M, Scherr DS, Lee RK, et al. Assessing the clinical benefit of nuclear matrix protein 22 in the surveillance of patients with nonmuscle-invasive bladder cancer and negative cytology: a decision-curve analysis. Cancer. 2011;117(13):2892–7. https://doi.org/10.1002/cncr.25903.
Shariat SF, Zippe C, Ludecke G, Boman H, Sanchez-Carbayo M, Casella R, et al. Nomograms including nuclear matrix protein 22 for prediction of disease recurrence and progression in patients with Ta, T1 or CIS transitional cell carcinoma of the bladder. J Urol. 2005;173(5):1518–25. https://doi.org/10.1097/01.ju.0000154696.48217.75.
Sanchez-Carbayo M, Herrero E, Megias J, Mira A, Soria F. Comparative sensitivity of urinary CYFRA 21-1, urinary bladder cancer antigen, tissue polypeptide antigen, tissue polypeptide antigen and NMP22 to detect bladder cancer. J Urol. 1999;162(6):1951–6.
Grossman HB, Soloway M, Messing E, Katz G, Stein B, Kassabian V, et al. Surveillance for recurrent bladder cancer using a point-of-care proteomic assay. JAMA. 2006;295(3):299–305. https://doi.org/10.1001/jama.295.3.299.
Grossman HB, Messing E, Soloway M, Tomera K, Katz G, Berger Y, et al. Detection of bladder cancer using a point-of-care proteomic assay. JAMA. 2005;293(7):810–6. https://doi.org/10.1001/jama.293.7.810.
Toma MI, Friedrich MG, Hautmann SH, Jakel KT, Erbersdobler A, Hellstern A, et al. Comparison of the ImmunoCyt test and urinary cytology with other urine tests in the detection and surveillance of bladder cancer. World J Urol. 2004;22(2):145–9. https://doi.org/10.1007/s00345-003-0390-8.
Mian C, Pycha A, Wiener H, Haitel A, Lodde M, Marberger M. Immunocyt: a new tool for detecting transitional cell cancer of the urinary tract. J Urol. 1999;161(5):1486–9.
Vriesema JL, Atsma F, Kiemeney LA, Peelen WP, Witjes JA, Schalken JA. Diagnostic efficacy of the ImmunoCyt test to detect superficial bladder cancer recurrence. Urology. 2001;58(3):367–71.
Pfister C, Chautard D, Devonec M, Perrin P, Chopin D, Rischmann P, et al. Immunocyt test improves the diagnostic accuracy of urinary cytology: results of a French multicenter study. J Urol. 2003;169(3):921–4. https://doi.org/10.1097/01.ju.0000048983.83079.4c.
Mian C, Maier K, Comploj E, Lodde M, Berner L, Lusuardi L, et al. uCyt+/ImmunoCyt in the detection of recurrent urothelial carcinoma: an update on 1991 analyses. Cancer. 2006;108(1):60–5. https://doi.org/10.1002/cncr.21712.
Olsson H, Zackrisson B. ImmunoCyt a useful method in the follow-up protocol for patients with urinary bladder carcinoma. Scand J Urol Nephrol. 2001;35(4):280–2.
Sokolova IA, Halling KC, Jenkins RB, Burkhardt HM, Meyer RG, Seelig SA, et al. The development of a multitarget, multicolor fluorescence in situ hybridization assay for the detection of urothelial carcinoma in urine. J Mol Diagn. 2000;2(3):116–23. https://doi.org/10.1016/S1525-1578(10)60625-3.
Hajdinjak T. UroVysion FISH test for detecting urothelial cancers: meta-analysis of diagnostic accuracy and comparison with urinary cytology testing. Urol Oncol. 2008;26(6):646–51. https://doi.org/10.1016/j.urolonc.2007.06.002.
Dimashkieh H, Wolff DJ, Smith TM, Houser PM, Nietert PJ, Yang J. Evaluation of urovysion and cytology for bladder cancer detection: a study of 1835 paired urine samples with clinical and histologic correlation. Cancer Cytopathol. 2013;121(10):591–7. https://doi.org/10.1002/cncy.21327.
Gofrit ON, Zorn KC, Silvestre J, Shalhav AL, Zagaja GP, Msezane LP, et al. The predictive value of multi-targeted fluorescent in-situ hybridization in patients with history of bladder cancer. Urol Oncol. 2008;26(3):246–9. https://doi.org/10.1016/j.urolonc.2007.02.011.
Skacel M, Fahmy M, Brainard JA, Pettay JD, Biscotti CV, Liou LS, et al. Multitarget fluorescence in situ hybridization assay detects transitional cell carcinoma in the majority of patients with bladder cancer and atypical or negative urine cytology. J Urol. 2003;169(6):2101–5. https://doi.org/10.1097/01.ju.0000066842.45464.cc.
Yoder BJ, Skacel M, Hedgepeth R, Babineau D, Ulchaker JC, Liou LS, et al. Reflex UroVysion testing of bladder cancer surveillance patients with equivocal or negative urine cytology: a prospective study with focus on the natural history of anticipatory positive findings. Am J Clin Pathol. 2007;127(2):295–301. https://doi.org/10.1309/ADJL7E810U1H42BJ.
Schlomer BJ, Ho R, Sagalowsky A, Ashfaq R, Lotan Y. Prospective validation of the clinical usefulness of reflex fluorescence in situ hybridization assay in patients with atypical cytology for the detection of urothelial carcinoma of the bladder. J Urol. 2010;183(1):62–7. https://doi.org/10.1016/j.juro.2009.08.157.
Kim PH, Sukhu R, Cordon BH, Sfakianos JP, Sjoberg DD, Hakimi AA, et al. Reflex fluorescence in situ hybridization assay for suspicious urinary cytology in patients with bladder cancer with negative surveillance cystoscopy. BJU Int. 2014;114(3):354–9. https://doi.org/10.1111/bju.12516.
Kipp BR, Karnes RJ, Brankley SM, Harwood AR, Pankratz VS, Sebo TJ, et al. Monitoring intravesical therapy for superficial bladder cancer using fluorescence in situ hybridization. J Urol. 2005;173(2):401–4. https://doi.org/10.1097/01.ju.0000149825.83180.a4.
Savic S, Zlobec I, Thalmann GN, Engeler D, Schmauss M, Lehmann K, et al. The prognostic value of cytology and fluorescence in situ hybridization in the follow-up of nonmuscle-invasive bladder cancer after intravesical Bacillus Calmette-Guerin therapy. Int J Cancer. 2009;124(12):2899–904. https://doi.org/10.1002/ijc.24258.
Lodde M, Mian C, Mayr R, Comploj E, Trenti E, Melotti R, et al. Recurrence and progression in patients with non-muscle invasive bladder cancer: prognostic models including multicolor fluorescence in situ hybridization molecular grading. Int J Urol. 2014;21(10):968–72. https://doi.org/10.1111/iju.12509.
Mukhtar S, Perry MJ. Future prospects for bladder cancer biomarkers. BJU Int. 2011;108(10):1541–3. https://doi.org/10.1111/j.1464-410X.2011.10642.x.
Stoeber K, Halsall I, Freeman A, Swinn R, Doble A, Morris L, et al. Immunoassay for urothelial cancers that detects DNA replication protein Mcm5 in urine. Lancet. 1999;354(9189):1524–5. https://doi.org/10.1016/S0140-6736(99)04265-8.
Stoeber K, Swinn R, Prevost AT, de Clive-Lowe P, Halsall I, Dilworth SM, et al. Diagnosis of genito-urinary tract cancer by detection of minichromosome maintenance 5 protein in urine sediments. J Natl Cancer Inst. 2002;94(14):1071–9.
•• Dudderidge T, Nabi G, Mom J, Umez-Eronini N, Hrouda D, Cresswell J, et al. A novel non-invasive aid for bladder cancer diagnosis: a prospective, multi-centre study to evaluate the ADXBLADDER test. Eur Urol Suppl. 2018;17(2):e1424. https://doi.org/10.1016/S1569-9056(18)31835-9 Abstract presentation at the 2018 Annual European Association of Urology Congress meeting reporting the sensitivity, specificity, and negative predictive value of ADXBLADDER, a novel protein-based assay detecting MCM5.
Allory Y, Beukers W, Sagrera A, Flandez M, Marques M, Marquez M, et al. Telomerase reverse transcriptase promoter mutations in bladder cancer: high frequency across stages, detection in urine, and lack of association with outcome. Eur Urol. 2014;65(2):360–6. https://doi.org/10.1016/j.eururo.2013.08.052.
• Descotes F, Kara N, Decaussin-Petrucci M, Piaton E, Geiguer F, Rodriguez-Lafrasse C, et al. Non-invasive prediction of recurrence in bladder cancer by detecting somatic TERT promoter mutations in urine. Br J Cancer. 2017;117(4):583–7. https://doi.org/10.1038/bjc.2017.210 Report that detection of TERT mutations, a genomic biomarker associated with most bladder cancers, has high sensitivity and specificity for detecting bladder cancer.
Ward DG, Baxter L, Gordon NS, Ott S, Savage RS, Beggs AD, et al. Multiplex PCR and next generation sequencing for the non-invasive detection of bladder cancer. PLoS One. 2016;11(2):e0149756. https://doi.org/10.1371/journal.pone.0149756.
Billerey C, Chopin D, Aubriot-Lorton MH, Ricol D, Gil Diez de Medina S, Van Rhijn B, et al. Frequent FGFR3 mutations in papillary non-invasive bladder (pTa) tumors. Am J Pathol. 2001;158(6):1955–9. https://doi.org/10.1016/S0002-9440(10)64665-2.
van Rhijn BW, Lurkin I, Radvanyi F, Kirkels WJ, van der Kwast TH, Zwarthoff EC. The fibroblast growth factor receptor 3 (FGFR3) mutation is a strong indicator of superficial bladder cancer with low recurrence rate. Cancer Res. 2001;61(4):1265–8.
Zuiverloon TC, van der Aa MN, van der Kwast TH, Steyerberg EW, Lingsma HF, Bangma CH, et al. Fibroblast growth factor receptor 3 mutation analysis on voided urine for surveillance of patients with low-grade non-muscle-invasive bladder cancer. Clin Cancer Res. 2010;16(11):3011–8. https://doi.org/10.1158/1078-0432.CCR-09-3013.
•• Kim YH, Yan C, Lee IS, Piao XM, Byun YJ, Jeong P, et al. Value of urinary topoisomerase-IIA cell-free DNA for diagnosis of bladder cancer. Investig Clin Urol. 2016;57(2):106–12. https://doi.org/10.4111/icu.2016.57.2.106 Study reporting that real-time PCR analysis of cell-free DNA for overexpression of TopoIIA, a genomic biomarker, had high sensitivity and specificity for detecting bladder cancer.
Kim EJ, Lee YS, Kim YJ, Kim MJ, Ha YS, Jeong P et al. Clinical implications and prognostic values of topoisomerase-II alpha expression in primary non-muscle-invasive bladder cancer. Urology. 2010;75(6):1516 e9–13. doi:https://doi.org/10.1016/j.urology.2009.08.055.
•• Ribal MJ, Mengual L, Lozano JJ, Ingelmo-Torres M, Palou J, Rodriguez-Faba O, et al. Gene expression test for the non-invasive diagnosis of bladder cancer: A prospective, blinded, international and multicenter validation study. Eur J Cancer. 2016;54:131–8. https://doi.org/10.1016/j.ejca.2015.11.003 Prospective study validating that the detection of the combination of IGF2 and MAGE-A3 had high sensitivity and specificity for detecting bladder cancer.
Mengual L, Ribal MJ, Lozano JJ, Ingelmo-Torres M, Burset M, Fernandez PL, et al. Validation study of a noninvasive urine test for diagnosis and prognosis assessment of bladder cancer: evidence for improved models. J Urol. 2014;191(1):261–9. https://doi.org/10.1016/j.juro.2013.06.083.
Knowles MA, Platt FM, Ross RL, Hurst CD. Phosphatidylinositol 3-kinase (PI3K) pathway activation in bladder cancer. Cancer Metastasis Rev. 2009;28(3–4):305–16. https://doi.org/10.1007/s10555-009-9198-3.
Dyrskjot L, Zieger K, Kissow Lildal T, Reinert T, Gruselle O, Coche T, et al. Expression of MAGE-A3, NY-ESO-1, LAGE-1 and PRAME in urothelial carcinoma. Br J Cancer. 2012;107(1):116–22. https://doi.org/10.1038/bjc.2012.215.
De Plaen E, Arden K, Traversari C, Gaforio JJ, Szikora JP, De Smet C, et al. Structure, chromosomal localization, and expression of 12 genes of the MAGE family. Immunogenetics. 1994;40(5):360–9.
•• Kavalieris L, O’Sullivan P, Frampton C, Guilford P, Darling D, Jacobson E, et al. Performance characteristics of a multigene urine biomarker test for monitoring for recurrent urothelial carcinoma in a multicenter study. J Urol. 2017;197(6):1419–26. https://doi.org/10.1016/j.juro.2016.12.010 Initial report that Cxbladder Monitor, a transcriptomic biomarker assay, had high sensitivity and specificity for detecting bladder cancer.
•• Pichler R, Fritz J, Tulchiner G, Klinglmair G, Soleiman A, Horninger W, et al. Increased accuracy of a novel mRNA-based urine test for bladder cancer surveillance. BJU Int. 2018;121(1):29–37. https://doi.org/10.1111/bju.14019 Initial study reporting the development of XPERT BC Monitor, a transcriptomic biomarker assay, that had a significantly superior sensitivity and negative predictive value for detection of bladder cancer compared to cytology.
•• Feber A, Dhami P, Dong L, de Winter P, Tan WS, Martinez-Fernandez M, et al. UroMark-a urinary biomarker assay for the detection of bladder cancer. Clin Epigenetics. 2017;9:8. https://doi.org/10.1186/s13148-016-0303-5 Initial report of the development and validation of UroMark, an epigenetic biomarker assay, demonstrating that UroMark had a high specificity and negative predictive value for the detection of primary bladder cancer.
• van Kessel KE, Van Neste L, Lurkin I, Zwarthoff EC, Van Criekinge W. Evaluation of an epigenetic profile for the detection of bladder cancer in patients with hematuria. J Urol. 2016;195(3):601–7. https://doi.org/10.1016/j.juro.2015.08.085 Initial report of the development of AssureMDX, an epigenetic biomarker assay, for the detection of bladder cancer.
•• van Kessel KE, Beukers W, Lurkin I, Ziel-van der Made A, van der Keur KA, Boormans JL, et al. Validation of a dna methylation-mutation urine assay to select patients with hematuria for cystoscopy. J Urol. 2017;197(3 Pt 1):590–5. https://doi.org/10.1016/j.juro.2016.09.118 Validation study of AssureMDX confirming high sensitivity, specificity, and negative predictive value reported in the initial development study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
William Tabayoyong declares no potential conflicts of interest.
Ashish M. Kamat reports grants and is a consultant/advisor for Abbott Molecular, Arquer Diagnostics, MDxHealth, Pacific Edge, Cepheid, and Cyprit.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Urothelial Cancer
Rights and permissions
About this article
Cite this article
Tabayoyong, W., Kamat, A.M. Current Use and Promise of Urinary Markers for Urothelial Cancer. Curr Urol Rep 19, 96 (2018). https://doi.org/10.1007/s11934-018-0857-1
Published:
DOI: https://doi.org/10.1007/s11934-018-0857-1