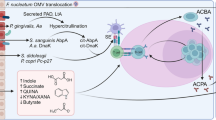
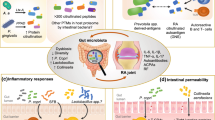
Abstract
Purpose of Review
This review will summarize recent data defining the relationship between rheumatoid arthritis (RA) and the microbiome at mucosal sites throughout the body. It will highlight what is known, what is speculated, and current knowledge gaps regarding the microbiome in RA.
Recent Findings
An extensive relationship between the microbiome and immune cell function can influence RA-related inflammation and T cell and B cell biology. Studies are beginning to characterize microbial changes in individuals who are at risk for RA, which is a critical element needed to understand the influence of the microbiome on RA pathogenesis.
Summary
Expanding our understanding of the microbiome in RA beyond the bacteria at the gut and oral mucosae into the lung and urogenital surfaces, including viral and fungal components, and establishing the relationship across mucosal sites will be critical in future work. Importantly, approaches to manipulate the microbiome could lead to novel therapeutic and preventive strategies.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–98. https://doi.org/10.1016/j.cell.2009.09.033.
Khosravi A, Yanez A, Price JG, Chow A, Merad M, Goodridge HS, et al. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe. 2014;15(3):374–81. https://doi.org/10.1016/j.chom.2014.02.006.
Kim D, Kim YG, Seo SU, Kim DJ, Kamada N, Prescott D, et al. Nod2-mediated recognition of the microbiota is critical for mucosal adjuvant activity of cholera toxin. Nat Med. 2016;22(5):524–30. https://doi.org/10.1038/nm.4075.
Makki K, Deehan EC, Walter J, Backhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23(6):705–15. https://doi.org/10.1016/j.chom.2018.05.012.
Maeda Y, Kurakawa T, Umemoto E, Motooka D, Ito Y, Gotoh K, et al. Dysbiosis contributes to arthritis development via activation of autoreactive T cells in the intestine. Arthritis Rheum. 2016;68(11):2646–61. https://doi.org/10.1002/art.39783.
Holers VM, Demoruelle MK, Kuhn KA, Buckner JH, Robinson WH, Okamoto Y, et al. Rheumatoid arthritis and the mucosal origins hypothesis: protection turns to destruction. Nat Rev Rheumatol. 2018;14(9):542–57. https://doi.org/10.1038/s41584-018-0070-0.
Eerola E, Mottonen T, Hannonen P, Luukkainen R, Kantola I, Vuori K, et al. Intestinal flora in early rheumatoid arthritis. Br J Rheumatol. 1994;33(11):1030–8. https://doi.org/10.1093/rheumatology/33.11.1030.
De Luca F, Shoenfeld Y. The microbiome in autoimmune diseases. Clin Exp Immunol. 2019;195(1):74–85. https://doi.org/10.1111/cei.13158.
Zhang X, Zhang D, Jia H, Feng Q, Wang D, Liang D, et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med. 2015;21(8):895–905. https://doi.org/10.1038/nm.3914.
Chen J, Wright K, Davis JM, Jeraldo P, Marietta EV, Murray J, et al. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 2016;8(1):43. https://doi.org/10.1186/s13073-016-0299-7.
Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013;2:e01202. https://doi.org/10.7554/eLife.01202.
•• Pianta A, Arvikar S, Strle K, Drouin EE, Wang Q, Costello CE, et al. Evidence of the Immune relevance of Prevotella copri, a gut microbe, in patients with rheumatoid arthritis. Arthritis Rheum. 2017;69(5):964–75. https://doi.org/10.1002/art.40003This study identified a potential mechanism by which P. copri peptides could contribute to RA pathogenesis through stimulation of Th1 responses in a subgroup of RA patients.
Nguyen Y, Mariette X, Salliot C, Gusto G, Boutron-Ruault MC, Seror R. Chronic diarrhoea and risk of rheumatoid arthritis: findings from the French E3N-EPIC cohort study. Rheumatology (Oxford). 2020. https://doi.org/10.1093/rheumatology/keaa133.
•• Alpizar-Rodriguez D, Lesker TR, Gronow A, Gilbert B, Raemy E, Lamacchia C, et al. Prevotella copri in individuals at risk for rheumatoid arthritis. Ann Rheum Dis. 2019;78(5):590–3. https://doi.org/10.1136/annrheumdis-2018-214514This is one of the first studies to characterize the gut microbiome in first degree relatives of those with RA in addition to a subset with preclinical RA, thereby opening the door to better understand the microbiota as a potential trigger of RA in humans.
Asquith M, Sternes PR, Costello ME, Karstens L, Diamond S, Martin TM, et al. HLA alleles associated with risk of Ankylosing spondylitis and rheumatoid arthritis influence the gut microbiome. Arthritis Rheum. 2019;71(10):1642–50. https://doi.org/10.1002/art.40917.
Klareskog L, Stolt P, Lundberg K, Kallberg H, Bengtsson C, Grunewald J, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54(1):38–46. https://doi.org/10.1002/art.21575.
Johansson K, Askling J, Alfredsson L, Di Giuseppe D, group Es. Mediterranean diet and risk of rheumatoid arthritis: a population-based case-control study. Arthritis Res Ther. 2018;20(1):175. https://doi.org/10.1186/s13075-018-1680-2.
Lee SH, Yun Y, Kim SJ, Lee EJ, Chang Y, Ryu S, et al. Association between cigarette smoking status and composition of gut microbiota: population-based cross-sectional study. J Clin Med. 2018;7(9):282. https://doi.org/10.3390/jcm7090282.
Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15(1):73. https://doi.org/10.1186/s12967-017-1175-y.
Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32(6):815–27. https://doi.org/10.1016/j.immuni.2010.06.001.
Rogier R, Ederveen THA, Boekhorst J, Wopereis H, Scher JU, Manasson J, et al. Aberrant intestinal microbiota due to IL-1 receptor antagonist deficiency promotes IL-17- and TLR4-dependent arthritis. Microbiome. 2017;5(1):63. https://doi.org/10.1186/s40168-017-0278-2.
Teng F, Klinger CN, Felix KM, Bradley CP, Wu E, Tran NL, et al. Gut microbiota drive autoimmune arthritis by promoting differentiation and migration of Peyer's patch T follicular helper cells. Immunity. 2016;44(4):875–88. https://doi.org/10.1016/j.immuni.2016.03.013.
Pietrosimone KM, Jin M, Poston B, Liu P. Collagen-Induced Arthritis: A model for Murine Autoimmune Arthritis. Bio Protoc. 2015;5(20):e1626. https://doi.org/10.21769/bioprotoc.1626.
Jubair WK, Hendrickson JD, Severs EL, Schulz HM, Adhikari S, Ir D, et al. Modulation of inflammatory arthritis in mice by gut microbiota through mucosal inflammation and autoantibody generation. Arthritis Rheum. 2018;70(8):1220–33. https://doi.org/10.1002/art.40490.
• Rosser EC, Piper CJM, Matei DE, Blair PA, Rendeiro AF, Orford M, et al. Microbiota-derived metabolites suppress arthritis by amplifying aryl-hydrocarbon receptor activation in regulatory B cells. Cell Metab. 2020;31(4):837–51 e10. https://doi.org/10.1016/j.cmet.2020.03.003This study demonstrated that supplementation of a known microbial-derived metabolite, butyrate, in mice significantly reduced arthritis by driving regulatory B cell populations and suppressing B cell and plasmablast differentiation.
Ascherman DP. Interstitial lung disease in rheumatoid arthritis. Curr Rheumatol Rep. 2010;12(5):363–9. https://doi.org/10.1007/s11926-010-0116-z.
Brito Y, Glassberg MK, Ascherman DP. Rheumatoid arthritis-associated interstitial lung disease: current concepts. Curr Rheumatol Rep. 2017;19(12):79. https://doi.org/10.1007/s11926-017-0701-5.
Spagnolo P, Lee JS, Sverzellati N, Rossi G, Cottin V. The lung in rheumatoid arthritis: focus on interstitial lung disease. Arthritis Rheum. 2018;70(10):1544–54. https://doi.org/10.1002/art.40574.
Willis VC, Demoruelle MK, Derber LA, Chartier-Logan CJ, Parish MC, Pedraza IF, et al. Sputum autoantibodies in patients with established rheumatoid arthritis and subjects at risk of future clinically apparent disease. Arthritis Rheum. 2013;65(10):2545–54. https://doi.org/10.1002/art.38066.
Demoruelle MK, Harrall KK, Ho L, Purmalek MM, Seto NL, Rothfuss HM, et al. Anti-citrullinated protein antibodies are associated with neutrophil extracellular traps in the sputum in relatives of rheumatoid arthritis patients. Arthritis Rheum. 2017;69(6):1165–75. https://doi.org/10.1002/art.40066.
Reynisdottir G, Karimi R, Joshua V, Olsen H, Hensvold AH, Harju A, et al. Structural changes and antibody enrichment in the lungs are early features of anti-citrullinated protein antibody-positive rheumatoid arthritis. Arthritis Rheum. 2014;66(1):31–9. https://doi.org/10.1002/art.38201.
Reynisdottir G, Olsen H, Joshua V, Engstrom M, Forsslund H, Karimi R, et al. Signs of immune activation and local inflammation are present in the bronchial tissue of patients with untreated early rheumatoid arthritis. Ann Rheum Dis. 2016;75(9):1722–7. https://doi.org/10.1136/annrheumdis-2015-208216.
Demoruelle MK, Weisman MH, Simonian PL, Lynch DA, Sachs PB, Pedraza IF, et al. Brief report: airways abnormalities and rheumatoid arthritis-related autoantibodies in subjects without arthritis: early injury or initiating site of autoimmunity? Arthritis Rheum. 2012;64(6):1756–61. https://doi.org/10.1002/art.34344.
Scher JU, Joshua V, Artacho A, Abdollahi-Roodsaz S, Ockinger J, Kullberg S, et al. The lung microbiota in early rheumatoid arthritis and autoimmunity. Microbiome. 2016;4(1):60. https://doi.org/10.1186/s40168-016-0206-x.
Huang C, Shi G. Smoking and microbiome in oral, airway, gut and some systemic diseases. J Transl Med. 2019;17(1):225. https://doi.org/10.1186/s12967-019-1971-7.
Morris A, Beck JM, Schloss PD, Campbell TB, Crothers K, Curtis JL, et al. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am J Respir Crit Care Med. 2013;187(10):1067–75. https://doi.org/10.1164/rccm.201210-1913OC.
Mammen MJ, Sethi S. COPD and the microbiome. Respirology. 2016;21(4):590–9. https://doi.org/10.1111/resp.12732.
Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–5. https://doi.org/10.1126/science.1092385.
Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5(178):178ra40. https://doi.org/10.1126/scitranslmed.3005580.
Carmona-Rivera C, Carlucci PM, Moore E, Lingampalli N, Uchtenhagen H, James E, et al. Synovial fibroblast-neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis. Sci Immunol. 2017;2(10):eaag3358. https://doi.org/10.1126/sciimmunol.aag3358.
Demoruelle MK, Bowers E, Lahey LJ, Sokolove J, Purmalek M, Seto NL, et al. Antibody responses to citrullinated and noncitrullinated antigens in the sputum of subjects with rheumatoid arthritis and subjects at risk for development of rheumatoid arthritis. Arthritis Rheum. 2018;70(4):516–27. https://doi.org/10.1002/art.40401.
Dickson RP, Martinez FJ, Huffnagle GB. The role of the microbiome in exacerbations of chronic lung diseases. Lancet. 2014;384(9944):691–702. https://doi.org/10.1016/S0140-6736(14)61136-3.
Salisbury ML, Han MK, Dickson RP, Molyneaux PL. Microbiome in interstitial lung disease: from pathogenesis to treatment target. Curr Opin Pulm Med. 2017;23(5):404–10. https://doi.org/10.1097/MCP.0000000000000399.
Han MK, Zhou Y, Murray S, Tayob N, Noth I, Lama VN, et al. Lung microbiome and disease progression in idiopathic pulmonary fibrosis: an analysis of the COMET study. Lancet Respir Med. 2014;2(7):548–56. https://doi.org/10.1016/S2213-2600(14)70069-4.
Molyneaux PL, Cox MJ, Wells AU, Kim HC, Ji W, Cookson WO, et al. Changes in the respiratory microbiome during acute exacerbations of idiopathic pulmonary fibrosis. Respir Res. 2017;18(1):29. https://doi.org/10.1186/s12931-017-0511-3.
O'Dwyer DN, Ashley SL, Gurczynski SJ, Xia M, Wilke C, Falkowski NR, et al. Lung microbiota contribute to pulmonary inflammation and disease progression in pulmonary fibrosis. Am J Respir Crit Care Med. 2019;199(9):1127–38. https://doi.org/10.1164/rccm.201809-1650OC.
Tong X, Su F, Xu X, Xu H, Yang T, Xu Q, et al. Alterations to the lung microbiome in idiopathic pulmonary fibrosis patients. Front Cell Infect Microbiol. 2019;9:149. https://doi.org/10.3389/fcimb.2019.00149.
Juge PA, Lee JS, Ebstein E, Furukawa H, Dobrinskikh E, Gazal S, et al. MUC5B promoter variant and rheumatoid arthritis with interstitial lung disease. N Engl J Med. 2018;379(23):2209–19. https://doi.org/10.1056/NEJMoa1801562.
Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364(16):1503–12. https://doi.org/10.1056/NEJMoa1013660.
Zhang Q, Wang Y, Qu D, Yu J, Yang J. The possible pathogenesis of idiopathic pulmonary fibrosis considering MUC5B. Biomed Res Int. 2019;2019:9712464–12. https://doi.org/10.1155/2019/9712464.
de Pablo P, Chapple IL, Buckley CD, Dietrich T. Periodontitis in systemic rheumatic diseases. Nat Rev Rheumatol. 2009;5(4):218–24. https://doi.org/10.1038/nrrheum.2009.28.
Dissick A, Redman RS, Jones M, Rangan BV, Reimold A, Griffiths GR, et al. Association of periodontitis with rheumatoid arthritis: a pilot study. J Periodontol. 2010;81(2):223–30. https://doi.org/10.1902/jop.2009.090309.
Fuggle NR, Smith TO, Kaul A, Sofat N. Hand to mouth: a systematic review and meta-analysis of the association between rheumatoid arthritis and periodontitis. Front Immunol. 2016;7:80. https://doi.org/10.3389/fimmu.2016.00080.
Mikuls TR, Payne JB, Yu F, Thiele GM, Reynolds RJ, Cannon GW, et al. Periodontitis and Porphyromonas gingivalis in patients with rheumatoid arthritis. Arthritis Rheum. 2014;66(5):1090–100. https://doi.org/10.1002/art.38348.
Correa JD, Fernandes GR, Calderaro DC, Mendonca SMS, Silva JM, Albiero ML, et al. Oral microbial dysbiosis linked to worsened periodontal condition in rheumatoid arthritis patients. Sci Rep. 2019;9(1):8379. https://doi.org/10.1038/s41598-019-44674-6.
Eriksson K, Fei G, Lundmark A, Benchimol D, Lee L, Hu YOO, et al. Periodontal health and oral microbiota in patients with rheumatoid arthritis. J Clin Med. 2019;8(5):630. https://doi.org/10.3390/jcm8050630.
Kaur S, Bright R, Proudman SM, Bartold PM. Does periodontal treatment influence clinical and biochemical measures for rheumatoid arthritis? A systematic review and meta-analysis. Semin Arthritis Rheum. 2014;44(2):113–22. https://doi.org/10.1016/j.semarthrit.2014.04.009.
Silvestre FJ, Silvestre-Rangil J, Bagan L, Bagan JV. Effect of nonsurgical periodontal treatment in patients with periodontitis and rheumatoid arthritis: a systematic review. Med Oral Patol Oral Cir Bucal. 2016;21(3):e349–54. https://doi.org/10.4317/medoral.20974.
Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nat Rev Microbiol. 2012;10(10):717–25. https://doi.org/10.1038/nrmicro2873.
Jung H, Jung SM, Rim YA, Park N, Nam Y, Lee J, et al. Arthritic role of Porphyromonas gingivalis in collagen-induced arthritis mice. PLoS One. 2017;12(11):e0188698. https://doi.org/10.1371/journal.pone.0188698.
Courbon G, Rinaudo-Gaujous M, Blasco-Baque V, Auger I, Caire R, Mijola L, et al. Porphyromonas gingivalis experimentally induces periodontis and an anti-CCP2-associated arthritis in the rat. Ann Rheum Dis. 2019;78(5):594–9. https://doi.org/10.1136/annrheumdis-2018-213697.
Lubcke PM, Ebbers MNB, Volzke J, Bull J, Kneitz S, Engelmann R, et al. Periodontal treatment prevents arthritis in mice and methotrexate ameliorates periodontal bone loss. Sci Rep. 2019;9(1):8128. https://doi.org/10.1038/s41598-019-44512-9.
Marchesan JT, Gerow EA, Schaff R, Taut AD, Shin SY, Sugai J, et al. Porphyromonas gingivalis oral infection exacerbates the development and severity of collagen-induced arthritis. Arthritis Res Ther. 2013;15(6):R186. https://doi.org/10.1186/ar4376.
Arvikar SL, Collier DS, Fisher MC, Unizony S, Cohen GL, McHugh G, et al. Clinical correlations with Porphyromonas gingivalis antibody responses in patients with early rheumatoid arthritis. Arthritis Res Ther. 2013;15(5):R109. https://doi.org/10.1186/ar4289.
• Hitchon CA, Chandad F, Ferucci ED, Willemze A, Ioan-Facsinay A, van der Woude D, et al. Antibodies to porphyromonas gingivalis are associated with anticitrullinated protein antibodies in patients with rheumatoid arthritis and their relatives. J Rheumatol. 2010;37(6):1105–12. https://doi.org/10.3899/jrheum.091323This study found that after oral inoculation of Pg, treatment with oral antibiotics prevented development of arthritis in CIA mice to the same degree as oral methotrexate.
Mikuls TR, Payne JB, Reinhardt RA, Thiele GM, Maziarz E, Cannella AC, et al. Antibody responses to Porphyromonas gingivalis (P. gingivalis) in subjects with rheumatoid arthritis and periodontitis. Int Immunopharmacol. 2009;9(1):38–42. https://doi.org/10.1016/j.intimp.2008.09.008.
Gomez-Banuelos E, Mukherjee A, Darrah E, Andrade F. Rheumatoid arthritis-associated mechanisms of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. J Clin Med. 2019;8(9):1309. https://doi.org/10.3390/jcm8091309.
Beyer K, Zaura E, Brandt BW, Buijs MJ, Brun JG, Crielaard W, et al. Subgingival microbiome of rheumatoid arthritis patients in relation to their disease status and periodontal health. PLoS One. 2018;13(9):e0202278. https://doi.org/10.1371/journal.pone.0202278.
Mikuls TR, Walker C, Qiu F, Yu F, Thiele GM, Alfant B, et al. The subgingival microbiome in patients with established rheumatoid arthritis. Rheumatology (Oxford). 2018;57(7):1162–72. https://doi.org/10.1093/rheumatology/key052.
Scher JU, Ubeda C, Equinda M, Khanin R, Buischi Y, Viale A, et al. Periodontal disease and the oral microbiota in new-onset rheumatoid arthritis. Arthritis Rheum. 2012;64(10):3083–94. https://doi.org/10.1002/art.34539.
• Tong Y, Zheng L, Qing P, Zhao H, Li Y, Su L, et al. Oral microbiota perturbations are linked to high risk for rheumatoid arthritis. Front Cell Infect Microbiol. 2019;9:475. https://doi.org/10.3389/fcimb.2019.00475This is one of the first studies to characterize the gingival microbiome in preclinical RA providing insight into how bacteria could have different effects at different stages of RA development.
Konig MF, Abusleme L, Reinholdt J, Palmer RJ, Teles RP, Sampson K, et al. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci Transl Med. 2016;8(369):369ra176. https://doi.org/10.1126/scitranslmed.aaj1921.
Volkov M, Dekkers J, Loos BG, Bizzarro S, Huizinga TWJ, Praetorius HA, et al. Comment on "Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis". Sci Transl Med. 2018;10(433):eaan8349. https://doi.org/10.1126/scitranslmed.aan8349.
Srinivasan S, Hoffman NG, Morgan MT, Matsen FA, Fiedler TL, Hall RW, et al. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One. 2012;7(6):e37818. https://doi.org/10.1371/journal.pone.0037818.
Ebringer A, Ptaszynska T, Corbett M, Wilson C, Macafee Y, Avakian H, et al. Antibodies to proteus in rheumatoid arthritis. Lancet. 1985;2(8450):305–7. https://doi.org/10.1016/s0140-6736(85)90352-6.
Ebringer A, Cunningham P, Ahmadi K, Wrigglesworth J, Hosseini R, Wilson C. Sequence similarity between HLA-DR1 and DR4 subtypes associated with rheumatoid arthritis and proteus/serratia membrane haemolysins. Ann Rheum Dis. 1992;51(11):1245–6. https://doi.org/10.1136/ard.51.11.1245.
Wilson C, Thakore A, Isenberg D, Ebringer A. Correlation between anti-proteus antibodies and isolation rates of P. mirabilis in rheumatoid arthritis. Rheumatol Int. 1997;16(5):187–9. https://doi.org/10.1007/BF01330294.
Sandberg ME, Bengtsson C, Klareskog L, Alfredsson L, Saevarsdottir S. Recent infections are associated with decreased risk of rheumatoid arthritis: a population-based case-control study. Ann Rheum Dis. 2015;74(5):904–7. https://doi.org/10.1136/annrheumdis-2014-206493.
Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955-2007. Arthritis Rheum. 2010;62(6):1576–82. https://doi.org/10.1002/art.27425.
van Teijlingen NH, Helgers LC, Zijlstra-Willems EM, van Hamme JL, Ribeiro CMS, Strijbis K, et al. Vaginal dysbiosis associated-bacteria Megasphaera elsdenii and Prevotella timonensis induce immune activation via dendritic cells. J Reprod Immunol. 2020;138:103085. https://doi.org/10.1016/j.jri.2020.103085.
Jespers V, Kyongo J, Joseph S, Hardy L, Cools P, Crucitti T, et al. A longitudinal analysis of the vaginal microbiota and vaginal immune mediators in women from sub-Saharan Africa. Sci Rep. 2017;7(1):11974. https://doi.org/10.1038/s41598-017-12198-6.
Hammad DBM, Hider SL, Liyanapathirana VC, Tonge DP. Molecular characterization of circulating microbiome signatures in rheumatoid arthritis. Front Cell Infect Microbiol. 2019;9:440. https://doi.org/10.3389/fcimb.2019.00440.
Manasson J, Blank RB, Scher JU. The microbiome in rheumatology: where are we and where should we go? Ann Rheum Dis. 2020;79(6):727–33. https://doi.org/10.1136/annrheumdis-2019-216631.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
MKD has received research support from Pfizer Inc. on studies related to RA. All other authors have declared that no conflict of interest exists.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Rheumatoid Arthritis
Rights and permissions
About this article
Cite this article
Wilson, T.M., Trent, B., Kuhn, K.A. et al. Microbial Influences of Mucosal Immunity in Rheumatoid Arthritis. Curr Rheumatol Rep 22, 83 (2020). https://doi.org/10.1007/s11926-020-00960-1
Published:
DOI: https://doi.org/10.1007/s11926-020-00960-1