Abstract
Purpose of Review
This article reviews the relationship of the microbiome, the gut-brain axis, and depression. It also will review factors which can influence this relationship, such as chronic stress, medications, and the Western diet typically consumed by adolescents.
Recent Findings
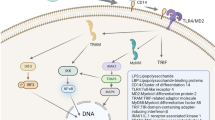
Changes in the gut microbiome increase the release of microbial lipopolysaccharides (LPS) which activate a gut inflammatory response. Gut pro-inflammatory cytokines stimulate the afferent vagal nerve which in turn impacts the hypothalamic-pituitary-adrenal (HPA) axis inducing symptoms associated with depression. Recent research suggests that gut inflammation can induce neuroinflammation which, in turn, stimulates microglia activation and the kynurenine pathway and can activate systemic inflammation-inducing depressive symptoms.
Summary
Promoting a healthy diet and lifestyle changes, limiting exposure to pesticides, limiting medications that affect the microbiome and the use of such things pre/probiotics and other interventions may complement existing efforts to curb the rise in depression. Alternative and complementary therapies may serve as effective treatments in adolescents with depression.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Twenge JM, Joiner TE, Rogers ML, Martin GN. Increases in depressive symptoms, suicide-related outcomes, and suicide rates among U.S. adolescents after 2010 and links to new media screen time. Clnical Psycho Sci. 2018;6(1):3–17.
Mojtabai R, Olfson M, Han B. National trends in the prevalence and treatment of depression in adolescents and young adults. Pediatrics. 2016; 138(6):
Lara GAG, Zuniga JO, Perez OC, Solís SH, Jiménez CEP, Méndez MC. Predictors of suicidal ideation and depressive symptoms in Chiapas, Mexico. Cien SaudeColet. 2018;23(4):1089–96.
Cipriani A, Zhou X, Del Giovane C, Hetrick SE, Qin B, Whittington C, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet. 2016;388:881–90. https://doi.org/10.1016/S0140-6736(16)30385-3.
Ignaszewski MJ, Waslick B. Update on randomized placebo-controlled trials in the past decade for treatment of major depressive disorder in child and adolescent patients: a systematic review. J Child Adolesc Psychopharmacol 2018 Jul 31. doi: https://doi.org/10.1089/cap.2017.0174. [Epub ahead of print], 28, 668, 675.
Messias E, Castro J, Saini A, Usman M, Peeples D. Sadness, suicide, and their association with video game and internet overuse among teens: results from the youth risk behavior survey 2007 and 2009. Suicide Life Threat Behav. 2011;41(3):307–15.
Carek PJ, Laibstain SE, Carek SM. Exercise for the treatment of depression and anxiety. Int J Psychiatry Med. 2011;41:15–28. https://doi.org/10.2190/PM.41.1.c.
Olweus D. Bullying at school: basic facts and effects of a school-based intervention program. J Child Psychol Psychiatry. 1994;35(7):1171–90.
Bailey MT, Coe CL. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev Psychobiol. 1999;2:146–55.
Dowlati Y, Herrmann N, Swardfager W. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–57. https://doi.org/10.1016/j.biopsych.2009.09.033.
Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–86.
Munkholm K, Braüner JV, Kessing LV, Vinberg M. Cytokines in bipolar disorder vs. healthy control subjects: a systematic review and meta-analysis. J Psychiatr Res. 2013;47(9):1119–33. https://doi.org/10.1016/j.jpsychires.
Gill J, Vythilingam M, Page GG. Low cortisol, high DHEA, and high levels of stimulated TNF-alpha, and IL-6 in women with PTSD. J Trauma Stress. 2008;21(6):530–9. https://doi.org/10.1002/jts.20372.
Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85–97.
Rethorst CD, Bernstein I, Trivedi MH. Inflammation, obesity, and metabolic syndrome in depression: analysis of the 2009-2010 National Health and Nutrition Examination Survey (NHANES). J Clin Psychiatry. 2014;75(12):e1428–32. https://doi.org/10.4088/JCP.14m09009.
Li Y, Lv MR, Wei YJ, Sun L, Zhang JX, Zhang HG, Li B Dietary patterns and depression risk: a meta-analysis. Psychiatry Res 2017 Jul;253:373–382. doi: https://doi.org/10.1016/j.psychres.2017.04.020. Epub 2017 Apr 11.
Mannan M, Mamun A, Doi S, Clavariano A. Prospective associations between depression and obesity for adolescent males and females-a systemic review and meta-analysis of longitudinal studies. PLoS One. 2016;11(6):e0157240.
Malik VS, Willett WC, Hu FB. Sugar-sweetened beverages and BMI in children and adolescents: re-analyses of a meta-analysis. Am J Clin Nutr. 2009;89:438–9.
Ebbeling CB, Feldman HA, Osganian SK, Chomitz VR, Ellenbogen SJ, Ludwig DS. Effects of decreasing sugar-sweetened beverage consumption on body weight in adolescents: a randomized, controlled pilot study. Pediatrics. 2006;117(3):673–80.
Jacka FN, O’Neil A, Opie R, Itsiopoulos C, Cotton S, et al. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med. 2017;15:23. https://doi.org/10.1186/s12916-017-0791-y.
•• Foster JA, Rinaman L, Cryan JF. Stress & the gut-brain axis: regulation by the microbiome. Neurobiol Stress. 2017;7:124–36. https://doi.org/10.1016/j.ynstr.2017.03.001. Clear review of the relationship to the gut-brain axis and also early causes for this disruption related to the vagal nerve.
•• Bonaz B, Bazin T, Pellissier S. The vagus nerve at the Interface of the microbiota-gut-brain axis. Front Neurosci. 2018;12:49. https://doi.org/10.3389/fnins.2018.00049. Important update on the role of the vagal nerve and the gut-brain axis.
•• Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392. https://doi.org/10.3389/fncel.2015.00392. One of the first important reviews of the gut-brain axis and its relationship to psychiatric disorders.
Schippa S, Conte MP. Dysbiotic events in gut microbiota: impact on human health. Nutrients. 2014;6(12):5786–805. https://doi.org/10.3390/nu6125786.
Round JL, Mazmanian SK. The gut microbiome shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9(5):313–23. https://doi.org/10.1038/nri2515.
Blaser MJ. The microbe revolution. J Clin Invest. 2014;124(10):4162–5.
•• Bonaz B, Bazin T, Pellister S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front Neurosci. 2018;12:49. https://doi.org/10.3389/fnins.2018.00049 eCollection 2018. Discusses the interplay between the vagal nerve and the gut.
Overman EL, Rivier JE, Moeser AJ. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-α. PLoS One 2012;7(6):e39935. doi: https://doi.org/10.1371/journal.pone.0039935. Epub 2012 Jun 29.
Valdez GR. Development of CRF1 receptor antagonists as antidepressants and anxiolytics: progress to date. CNS Drugs. 2006;20(11):887–96.
Wallace C, Milev R. The effects of probiotics on depressive symptoms in humans: a systematic review. Ann General Psychiatry. 2017;16:14. https://doi.org/10.1186/s12991-017-0138-2.
Liu Y, Ho RC, Mak AJ Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. Affect Disord 2012;139(3):230–239. doi: https://doi.org/10.1016/j.jad.2011.08.003.
Felger J, Harron E, Patel TA, Goldsmith DR, Wommack EC, et al. What does plasma CRP tell us about peripheral and central inflammation in depression? Mol Psychiatry. https://doi.org/10.1038/s41380-018-0096-3.
Freed RD, Mehra LM, Laor D, Patel M, Alonso CM, et al. Anhedonia as a clinical correlate of inflammation in psychiatric disorders. World J Biol Psychiatry 2018; 30:1–35.
Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, et al. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry. 2016;10:1358–65. https://doi.org/10.1038/mp.2015.168.
Karlović D, Serretti A, Vrkić N, Martinac M, Marčinko D. Serum concentrations of CRP, IL-6, TNF-α and cortisol in major depressive disorder with melancholic or atypical features. Psychiatry Res. 2012 Jun 30;198(1):74–80. https://doi.org/10.1016/j.psychres.2011.12.007.
Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36(5):305–12.
• Maes M, Berk M, Goehler L, Song C, Anderson G, et al. Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med. 2012;10:66. https://doi.org/10.1186/1741-7015-10-66. Review. A good review of the effect of cytokines on neuroinflammation and the overstimulation of microglia to develop “sickness behavior.
Bi W, Zhu L, Jing X, Zeng Z, Liang Y, et al. Rifampicin improves neuronal apoptosis in LPS-stimulated co-cultured BV2 cells through inhibition of the TLR-4 pathway. Mol Med Rep. 2014;10(4):1793–9.
DiSabato DJ, Quan N, Godbout JP. Neuroinflammation: the devil is in the details. J Neurochem 2016;139 Suppl 2:136–153. doi: https://doi.org/10.1111/jnc.13607. Epub 2016 May 4.
• Barreto FS, Chaves Filho AJM, de Araújo MCCR, de Moraes MO, de Moraes MEA, et al. Tryptophan catabolites along the indoleamine 2,3-dioxygenase pathway as a biological link between depression and cancer. Behav Pharmacol. 2018;29(2 and 3 - Special Issue):165–80. https://doi.org/10.1097/FBP.0000000000000384. Article describes the effect of inflammation on the IDO pathway.
• Swardfager W, Herrmann N, Dowlati Y, Oh PI, Kiss A ,et al. Indoleamine 2,3-dioxygenase activation and depressive symptoms in patients with coronary artery disease. Psychoneuroendocrinology. 2009;34(10):1560–1566. doi: https://doi.org/10.1016/j.psyneuen.2009.05.019. Epub 2009 Jun 21. Effect of inflammation on the IDO pathway and association with depression.
Richardson AS, Dietz WH, Gordon-Larsen P. The association between childhood sexual and physical abuse with incident adult severe obesity across 13 years of the National Longitudinal Study of Adolescent Health. Pediatr Obes 2014 Oct;9(5):351–361. doi: https://doi.org/10.1111/j.2047-6310.2013.00196.x. Epub 2013 Sep 20.
Costa CS, Del-Ponte B Assuncao MCF, Santos IS. Consumption of ultra-processed foods and body fat during childhood and adolescence: a systematic review. Public Health Nutr 2018;21(1):148–159. doi: https://doi.org/10.1017/S1368980017001331. Epub 2017 Jul 5.
Sánchez-Villegas A, Toledo E, de Irala J, Ruiz-Canela M, Pla-Vidal J, et al. Fast-food and commercial baked goods consumption and the risk of depression. Public Health Nutr. 2012;15:424–32. https://doi.org/10.1017/S1368980011001856.
Moreira AP, Texeira TF, Ferreira AB, PeluzioMdo C, Alfenas RC. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br JNutr. 2012;108:801–9. https://doi.org/10.1017/S0007114512001213.
Ghanim H, Abuaysheh S, Sia CL, Korzeniewski K, Chaudhuri A, Fernandez-real JM, et al. Increase in plasma endotoxin concentrations and the expression of toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. DiabetesCare. 2009;32:2281–7. https://doi.org/10.2337/dc09-0979.
Fatima Y, Doi SA, Mamun AA. Longitudinal impact of sleep on overweight and obesity in children and adolescents: a systematic review and bias-adjusted meta-analysis. Obes Rev. 2015;16(2):137–49. https://doi.org/10.1111/obr.12245.
Trandafir LM, Temneanu OR. Pre and post-natal risk and determination of factors for child obesity. J Med Life. 2016;9(4):386–91.
Sartorius N. Depression and diabetes. Dialogues Clin Neurosci. 2018;20(1):47–52.
Karra E, Chandarana K, Batterham RL. The role of peptide YY in appetite regulation and obesity. J Physiol. 2009;587:19–25. https://doi.org/10.1113/jphysiol.2008.164269.
Steinert R, Beglinger C, Langhans W. Intestinal GLP-1 and satiation: from man to rodents and back. Int J Obes. 2016;40:198–205. https://doi.org/10.1038/ijo.2015.172.
Mobbs CV, Isoda F, Makimura H, Mastaitis J, Mizuno T, et al. Impaired glucose signaling as a cause of obesity and the metabolic syndrome: the glucoadipostatic hypothesis. Physiol Behav. 2005;85(1):3–23.
Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A randomized controlled trial of tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammation biomarkers. JAMA Psychiatry. 2013;70(1):31–41.
Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514(7521):181–6. https://doi.org/10.1038/nature13793.
Shell ER. Artificial sweeteners get a gut check scientific. American. 2015;312(4):32–4. https://doi.org/10.1038/scientificamerican0415-32.
Hillemacher T, Bachmann O, Kahl KG, Frieling H. Alcohol, microbiome, and their effect on psychiatric disorders. Prog Neuro-Psychopharmacol Biol Psychiatry. 2018;85:105–15. https://doi.org/10.1016/j.pnpbp.2018.04.015.
Leclercq S, Matamoros S, Cani PD, Neyrinck AM, Jamar F. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A. 2014;111(42):E4485–93. https://doi.org/10.1073/pnas.1415174111).
Strasburger VC. Children, adolescents, obesity, and the media. Pediatrics. 2011;128:201–8.
Pan W, Kastin AJ. Leptin: a biomarker for sleep disorders? Sleep Med Rev. 2014;18(3):283–90.
Yeh HW, Chien WC, Chung CH, Hu JM, Tzeng NS. Risk of psychiatric disorders in irritable bowel syndrome-a nationwide, population-based, cohort study. Int J Clin Pract. 2018;19:e13212. https://doi.org/10.1111/ijcp.13212.
Daulatzai MA. Non-celiac gluten sensitivity triggers gut dysbiosis, neuroinflammation, gut-brain axis dysfunction, and vulnerability for dementia. CNS Neurol Disord Drug Targets. 2015;14(1):110–31.
Genuis SJ and Lobo RA. Gluten sensitivity presenting as a neuropsychiatric disorder. 2014;2014:293206. doi: https://doi.org/10.1155/2014/293206..
Lionetti E, Leonardi S, Franzonello C, Mancardi M, Ruggiero M, et al. Gluten psychosis: confirmation of a new clinical entity. Nutrients. 2015;7(7):5532–9. https://doi.org/10.3390/nu7075235.
Freire C, Koifman S. Pesticides, depression and suicide: a systematic review of the epidemiological evidence. Int J Hyg Environ Health. 2013;216(4):445–60. https://doi.org/10.1016/j.ijheh.2012.12.003.
• Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol. 2011;14(1):123–30. https://doi.org/10.1017/S1461145710000805. Decreased levels of glutathione associated with oxidized stress is associated with depression.
• Singh NK, Banerjee BD, Bala K, Basu M, Chhillar N. Polymorphism in cytochrome P450 2D6, glutathione S-transferases Pi 1 genes, and organochlorine pesticides in Alzheimer disease: A case-control study in north Indian population. J Geriatr Psychiatry Neurol. 2014;27(2):119–27. https://doi.org/10.1177/0891988714522698. Slow metabolizers of cytochrome P450 2D6 may prevent the breakdown of pesticides which may lead to neuropsychiatric disorders.
D’Brant J. GMOs, gut flora, the shíkimate pathway and cytochrome dysregulation. Nutri Perspect: J Council Nutri Am Chiropract Assoc. 2014;37(1):5–12.
Murphy MP. Mitochondrial thiols in antioxidant protection and redox signaling: distinct roles for glutathionylation and other thiol modifications. Antioxid Redox Signal. 2011;16:476–95.
Rezin GT, Amboni G, Zugno AI, Quevedo J, Streck EL Mitochondrial dysfunction and psychiatric disorders. Neurochem Res 2009 Jun;34(6):1021–1029. doi: https://doi.org/10.1007/s11064-008-9865-8. Epub 2008 Nov 1.
Rosa AR, Singh N, Whitaker E, de Brito M, Lewis AM, Vieta E, Churchill GC, Geddes JR, Goodwin GM Altered plasma glutathione levels in bipolar disorder indicates higher oxidative stress; a possible risk factor for illness onset despite normal brain-derived neurotrophic factor (BDNF) levels. Psychol Med 2014 Aug;44(11):2409–2418. doi: https://doi.org/10.1017/S0033291714000014. Epub 2014 Jan 27.
• Berk M, Copolov DL, Dean O, et al. N-acetylcyteine for depressive symptoms in bipolar disorder-a double blind randomized placebo-controlled trial. Biol Psychiatry. 2008;64:468–75. The use of N -acetylcysteine is useful for the treatment of bipolar disorder.
Tomko RL, Gilmore AK, Gray KM. The role of depressive symptoms in treatment of adolescent cannabis use disorder with N-acetylcysteine. Addict Behav 2018 May 21;85:26–30. doi: https://doi.org/10.1016/j.addbeh.2018.05.014. [Epub ahead of print].
Lurie I, Yang YX, Haynes K, Mamtani R, Boursi B. Antibiotic exposure and the risk for depression, anxiety, or psychosis: a nested case-control study. J Clin Psychiatry. 2015;76(11):1522–8. https://doi.org/10.4088/JCP.15m09961.
Le Bastard Q, Al-Ghalith GA, Bregoire M, et al. Systematic review: human gut dysbiosis induced by non-antibiotic prescription medications. Aliment Pharmacol Ther. 2018;47(3):332–45. https://doi.org/10.1111/apt.14451.
Bahr SM, Tyler BC, Wooldridge N, Butcher BD, Burns TL, Teesch LM, et al. Use of the second-generation antipsychotic, risperidone, and secondary weight gain are associated with an altered gut microbiota in children. Transl Psychiatry. 2015 Oct 6;5:e652. https://doi.org/10.1038/tp.2015.135.
Nurmi, EL. Do microbiome-bile acid interactions explain antipsychotic-induced weight gain. Presented in a symposium on The Life Within: The unconscious contributions of gut microbiota to our lives. American Academy of Child and Adolescent Psychiatry Annual Meeting, Seattle, Washington. October 25th, 2018, unpublished.
Chamberlain SR, Cavanaugh J, de Boer P et al. Treatment-resistant depression and peripheral C-reactive protein 2018;16 May: 1–9 https://doi.org/10.1192/bjp.2018.66
Gur TL, Worly BL, & Bailey MT. Stress and the commensal microbiota: importance in parturition and infant neurodevelopment. Front Psychiatry, 2015;6(5). https://doi.org/10.3389/fpsyt.2015.00005.
Ahmadizar F, Vijverberg SJH, Arets HGM, de Boer A, Lang JE, Garssen J, et al. Early-life antibiotic exposure increases the risk of developing allergic symptoms later in life: a meta-analysis. Allergy. 2018 May;73(5):971–86. https://doi.org/10.1111/all.13332.
Kuo CH, Huang CH, Yang SN, Lee MS, Hung CH. Early life exposure to antibiotics and the risk of childhood allergic diseases: an update from the perspective of the hygiene hypothesis. J Microbiol Immunol Infect. 2013;46(5):320–9.
Biasucci G, Rubini M, Riboni S, Morelli L, Bessi E, Retetangos C. Mode of delivery affects the bacterial community in the newborn gut. Early Hum Dev. 2010;86(Suppl 1):13–5. https://doi.org/10.1016/j.earlhumdev.2010.01.004.
Barrett E, Guinane CM, Ryan CA, Dempsey EM, Murphy BP, O'Toole PW, et al. Microbiota diversity and stability of the preterm neonatal ileum and colon of two infants. Microbiome Open. 2013;2:215–25.
Weber M, Grote V, Closa-Monasterolo R, et al. Lower protein content in infant formula reduces BMI and obesity risk at school age: follow-up of a randomized trial. Am J ClinNutr. 2014;99(5):1041–51.
Lucas A, Boyes S, Bloom R, Aynsley-Green A. Metabolic and endocrine responses to a milk feed in six-day-old term infants: differences between breast and cow’s milk formula feeding. Actapaediatrica Scandinavica. 1981;70:195–200.
Isolauri E, Kalliomäki M, Laitinen K, Salminen S. Modulation of the maturing gut barrier and microbiota: a novel target in allergic disease. Curr Pharm Des. 2008;14(14):1368–75.
• Pärtty A, Kalliomäki M, Wacklin P, Salminen S, Isolauri E.A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: a randomized trial. Pediatr Res. 2015;77(6):823–828. doi: https://doi.org/10.1038/pr.2015.51. 11. One of the first studies to show that early use of probiotics is associated with lower risk for psychiatric disorders in youth.
Stratiki Z, Costalos C, et al. The effect of a bifidobacter supplemented bovine milk on intestinal permeability of preterm infants. Early Hum Dev. 2007;83:575–9. https://doi.org/10.1016/j.earlhumdev.2006.12.002.
Aaronson ST, Carpenter LL, Conway CR, Reimherr FW, Lisanby SH, Schwartz TL, et al. Vagus nerve stimulation therapy randomized to different amounts of electrical charge for treatment-resistant depression: acute and chronic effects. Brain Stimul. 2013;6:631–40.
Ng QX, Peters C, Ho CYX, Lim DY, Yeo WS. A meta-analysis of the use of probiotics to alleviate depressive symptoms. J Affect Disord. 2018 Mar 1;228:13–9. https://doi.org/10.1016/j.jad.2017.11.063.
• Wallace CJK, Milev R. The effects of probiotics on depressive symptoms in humans: a systematic review. Ann General Psychiatry. 2017;16:14. https://doi.org/10.1186/s12991-017-0138-2. Review of DBPCT that show positive effects of using probiotics for depression.
Messaoudi M, Lalonde R, Violle N. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. 2011;105:755–64.
Messaoudi M, Violle N, Bisson JF, Desor D, Javelot H, Rougeot C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes. 2011;2:256–61.
Steenbergen L, Sellaro R, van Hemert S, et al. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav Immun. 2015;48:258–64. https://doi.org/10.1016/j.bbi.2015.04.003.
Akkasheh G, Kashani-Poor Z, Tajabadi-Ebrahimi M, Jafari P, Akbari H, Taghizadeh M, et al. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. Nutrition. 2016;32:315–20.
Stetler C, Miller GE. Depression and hypothalamic–pituitary–adrenal activation: a quantitative summary of four decades of research. Psychosom Med. 2011;73(2):114–26.
Arnold, LE Placebo controlled Pilot data for three complementary/alternative treatments in autism. Presented in an Honors Presentation. American Academy of Child and Adolescent Psychiatry Annual Meeting, Seattle, Washington. October 25th, 2018, unpublished.
Luna, RA. Multiomic profiling of ASD: linking the gut mocrobiome and metabolome with behavior and gastrointestinal symptoms. Presented in a symposium on The Life Within: The unconscious contributions of gut microbiota to our lives. American Academy of Child and Adolescent Psychiatry Annual Meeting, Seattle, Washington. October 25th, 2018, unpublished.
Luoto R, Kalliomaki M, Laitinen K, Isolauri E. The impact of peri natal probiotic intervention on the development of overweight and obesity: follow up from birth to 10 years. Int J Obes. 2010;34:1531–7.
Salazar N, Dewulf EM, Neyrinck AM, Bindels LB, Cani PD, et al. Inulin-type fructans modulater intestinal Bifodobacterium species populations and decrease short-chain fatty acids in obese women. Clin Nutr. 2015;34:493–501.
Dahiya DK, Puniya M, Shandilya UK, Shandilya UK, Dhewa T, et al. Gut microbiota modulation and its relationship with obesity using prebiotic fibers and probiotics: a review. Front Microbiol. 2017;8:563. https://doi.org/10.3389/fmicb.2017.00563.eCollection2017.
Cepeda MS, Katz EG, Blacketer C. Microbiome-gut-brain axis: probiotics and their association with depression. J Neuropsychiatr Clin Neurosci. 2017;29(1):39–44. https://doi.org/10.1176/appi.neuropsych.15120410.
Benno Y, Suzuki K, Suzuki K, Narisawa K, Bruce WR, Mitsuoka T. Comparison of fecal microflora in rural Japan and urban Canadians. Microbiol Immunol. 1986;30:521–32.
Aguayo SV, de la Calderon Barca AM. Old fashioned vs. ultra-processed-based current diets: possible implication in the increased susceptibility to type 1 diabetes and celiac disease in childhood. Foods. 2017;6(11):100.
Gomes C, Martinho FC, Barbosa DS, Antunes LS, Póvoa HCC, et al. Increased root canal endotoxin levels are associated with chronic apical periodontitis, increased oxidative and nitrosative stress, major depression, severity of depression, and a lowered quality of life. Mol Neurobiol. 2018;55(4):2814–27. https://doi.org/10.1007/s12035-017-0545-z.
Eby GA 3rd, Eby KL. Magnesium for treatment-resistant depression: a review and hypothesis. Med Hypotheses. 2010;74(4):649–60. https://doi.org/10.1016/j.mehy.2009.10.051.
Milaneschi Y, Hoogendijk W, Lips P, Heijboer AC, Schoevers R, van Hemert AM, Beekman ATF, Smit JH, Penninx BWJH The association between low vitamin D and depressive disorders. Mol Psychiatry 2014;19(4):444–451. https://doi.org/10.1038/mp.2013.36. Epub 2013 Apr 9. PMID:23568194.
Swardfager W, Herrmann N, Mazereeuw G, Lanctôt KL. Reply to: serum zinc and the risk of depression in men: observations from a 20-year follow-up study. Biol Psychiatry. 2015;77(3):e13–4. https://doi.org/10.1016/j.biopsych.2014.06.006.
• Fristad MA, Young AS, Vesco AT, Nader ES, Healy KZ, et al. A randomized controlled trial of individual family psychoeducational psychotherapy & omega-3 fatty acids in youth with subsyndromal bipolar disorder. J Child Adolescent Psychopharmacol. 2015;25(10):764–74. https://doi.org/10.1089/cap.2015.0132. Demonstrates usefulness of omega-3 fatty acids as an adjunctive treatment for depression.
• Arnold LE, Young A, Belury MA, Cole RM, Gracious B, et al. Omega-3 fatty acid plasma levels before and after supplementation: correlations with mood and clinical outcomes in the Omega 3 and Therapy Studies (OATS). J Child & Adolescent Psychopharmacol. 2017;27(3):223–33. https://doi.org/10.1089/cap.2016.0123. Demonstrates usefulness of omega-3 fatty acids as an adjunctive treatment for depression.
Acknowledgments
I would like to thank Chadi Calarge, M.D., for reviewing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares that she has no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Child and Adolescent Disorders
Rights and permissions
About this article
Cite this article
Simkin, D.R. Microbiome and Mental Health, Specifically as It Relates to Adolescents. Curr Psychiatry Rep 21, 93 (2019). https://doi.org/10.1007/s11920-019-1075-3
Published:
DOI: https://doi.org/10.1007/s11920-019-1075-3