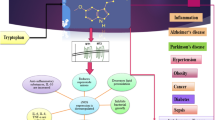
Abstract
The hypocretins (Hcrts), also known as orexins, have been among the most intensely studied neuropeptide systems since their discovery about two decades ago. Anatomical evidence shows that the hypothalamic neurons that produce hypocretins/orexins project widely throughout the entire brain, innervating the noradrenergic locus coeruleus, the cholinergic basal forebrain, the dopaminergic ventral tegmental area, the serotonergic raphe nuclei, the histaminergic tuberomammillary nucleus, and many other brain regions. By interacting with other neural systems, the Hcrt system profoundly modulates versatile physiological processes including arousal, food intake, emotion, attention, and reward. Importantly, interruption of the interactions between these systems has the potential to cause neurological and psychiatric diseases. Here, we review the modulation of diverse neural systems by Hcrts and summarize potential therapeutic strategies based on our understanding of the Hcrt system’s role in physiology and pathophysiological processes.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
de Lecea L, Kilduff TS, Peyron C, Gao XB, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95(1):322–7.
Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior (vol 92, pg 573, 1998). Cell. 1998;92(5):U29-U.
Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827(1–2):243–60.
Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18(23):9996–10015.
Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, et al. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46(2):297–308.
Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience. 2001;103(3):777–97.
Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438(1–2):71–5.
Zarepour L, Fatahi Z, Sarihi A, Haghparast A. Blockade of orexin-1 receptors in the ventral tegmental area could attenuate the lateral hypothalamic stimulation-induced potentiation of rewarding properties of morphine. Neuropeptides. 2014;48(3):179–85.
Balcita-Pedicino JJ, Sesack SR. Orexin axons in the rat ventral tegmental area synapse infrequently onto dopamine and gamma-aminobutyric acid neurons. J Comp Neurol. 2007;503(5):668–84.
Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437(7058):556–9.
Hutcheson DM, Quarta D, Halbout B, Rigal A, Valerio E, Heidbreder C. Orexin-1 receptor antagonist SB-334867 reduces the acquisition and expression of cocaine-conditioned reinforcement and the expression of amphetamine-conditioned reward. Behav Pharmacol. 2011;22(2):173–81.
James MH, Charnley JL, Levi EM, Jones E, Yeoh JW, Smith DW, et al. Orexin-1 receptor signalling within the ventral tegmental area, but not the paraventricular thalamus, is critical to regulating cue-induced reinstatement of cocaine-seeking. Int J Neuropsychopharmacol / Off Sci J Coll Int Neuropsychopharmacologicum. 2011;14(5):684–90.
Bentzley BS, Aston-Jones G. Orexin-1 receptor signaling increases motivation for cocaine-associated cues. Eur J Neurosci. 2015;41(9):1149–56.
Uslaner JM, Winrow CJ, Gotter AL, Roecker AJ, Coleman PJ, Hutson PH, et al. Selective orexin 2 receptor antagonism blocks cue-induced reinstatement, but not nicotine self-administration or nicotine-induced reinstatement. Behav Brain Res. 2014;269:61–5.
Martin-Fardon R, Weiss F. Blockade of hypocretin receptor-1 preferentially prevents cocaine seeking: comparison with natural reward seeking. Neuroreport. 2014;25(7):485–8.
Hollander JA, Pham D, Fowler CD, Kenny PJ. Hypocretin-1 receptors regulate the reinforcing and reward-enhancing effects of cocaine: pharmacological and behavioral genetics evidence. Front Behav Neurosci. 2012;6:47.
Baimel C, Borgland SL. Orexin signaling in the VTA gates morphine-induced synaptic plasticity. J Neurosci. 2015;35(18):7295–303.
Prince CD, Rau AR, Yorgason JT, Espana RA. Hypocretin/orexin regulation of dopamine signaling and cocaine self-administration is mediated predominantly by hypocretin receptor 1. ACS Chem Neurosci. 2015;6(1):138–46.
Baimel C, Borgland SL. Hypocretin modulation of drug-induced synaptic plasticity. Prog Brain Res. 2012;198:123–31.
Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49(4):589–601.
Borgland SL, Storm E, Bonci A. Orexin B/hypocretin 2 increases glutamatergic transmission to ventral tegmental area neurons. Eur J Neurosci. 2008;28(8):1545–56.
Muschamp JW, Hollander JA, Thompson JL, Voren G, Hassinger LC, Onvani S, et al. Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc Natl Acad Sci U S A. 2014;111(16):E1648–55. In this study, Hcrt was shown to facilitate reward by counteracting the antireward effects of dynorphin, which is released from the same synaptic vesicles in the VTA.
Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111(2):379–87.
Espana RA, Melchior JR, Roberts DC, Jones SR. Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacology. 2011;214(2):415–26.
Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, et al. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;102(52):19168–73.
Baimel C, Bartlett SE, Chiou LC, Lawrence AJ, Muschamp JW, Patkar O, et al. Orexin/hypocretin role in reward: implications for opioid and other addictions. Br J Pharmacol. 2015;172(2):334–48.
Brown RE, Sergeeva OA, Eriksson KS, Haas HL. Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/hypocretin, histamine and noradrenaline). J Neurosci. 2002;22(20):8850–9.
Kohlmeier KA, Tyler CJ, Kalogiannis M, Ishibashi M, Kristensen MP, Gumenchuk I, et al. Differential actions of orexin receptors in brainstem cholinergic and monoaminergic neurons revealed by receptor knockouts: implications for orexinergic signaling in arousal and narcolepsy. Front Neurosci. 2013;7:246.
Liu RJ, van den Pol AN, Aghajanian GK. Hypocretins (orexins) regulate serotonin neurons in the dorsal raphe nucleus by excitatory direct and inhibitory indirect actions. J Neurosci. 2002;22(21):9453–64.
Hasegawa E, Yanagisawa M, Sakurai T, Mieda M. Orexin neurons suppress narcolepsy via 2 distinct efferent pathways. J Clin Invest. 2014;124(2):604–16. This paper shows DR serotonergic and LC noradrenergic neurons represent different facets of gating narcoleptic symptoms.
Muraki Y, Yamanaka A, Tsujino N, Kilduff TS, Goto K, Sakurai T. Serotonergic regulation of the orexin/hypocretin neurons through the 5-HT1A receptor. J Neurosci. 2004;24(32):7159–66.
Carter ME, Brill J, Bonnavion P, Huguenard JR, Huerta R, de Lecea L. Mechanism for hypocretin-mediated sleep-to-wake transitions. Proc Natl Acad Sci U S A. 2012;109(39):E2635–44. Using optogenetic manipulation of Hcrt neurons in lateral hypothalamus and noradrenergic neurons in locus coeruleus (LC), this article demonstrates that the Hcrt-LC circuit is critical for tuning sleep-to-wake transitions.
Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13(12):1526–U117.
Mochizuki T, Crocker A, McCormack S, Yanagisawa M, Sakurai T, Scammell TE. Behavioral state instability in orexin knock-out mice. J Neurosci. 2004;24(28):6291–300.
Tsujino N, Tsunematsu T, Uchigashima M, Konno K, Yamanaka A, Kobayashi K, et al. Chronic alterations in monoaminergic cells in the locus coeruleus in orexin neuron-ablated narcoleptic mice. PLoS One. 2013;8(7), e70012.
Torrealba F, Yanagisawa M, Saper CB. Colocalization of orexin a and glutamate immunoreactivity in axon terminals in the tuberomammillary nucleus in rats. Neuroscience. 2003;119(4):1033–44.
Eriksson KS, Sergeeva O, Brown RE, Haas HL. Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci. 2001;21(23):9273–9.
Schöne C, Cao ZF, Apergis-Schoute J, Adamantidis A, Sakurai T, Burdakov D. Optogenetic probing of fast glutamatergic transmission from hypocretin/orexin to histamine neurons in situ. J Neurosci. 2012;32(36):12437–43.
Bassetti CL, Baumann CR, Dauvilliers Y, Croyal M, Robert P, Schwartz JC. Cerebrospinal fluid histamine levels are decreased in patients with narcolepsy and excessive daytime sleepiness of other origin. J Sleep Res. 2010;19(4):620–3.
Valko PO, Gavrilov YV, Yamamoto M, Finn K, Reddy H, Haybaeck J, et al. Damage to histaminergic tuberomammillary neurons and other hypothalamic neurons with traumatic brain injury. Ann Neurol. 2015;77(1):177–82.
Valko PO, Gavrilov YV, Yamamoto M, Reddy H, Haybaeck J, Mignot E, et al. Increase of histaminergic tuberomammillary neurons in narcolepsy. Ann Neurol. 2013;74(6):794–804.
Eggermann E, Serafin M, Bayer L, Machard D, Saint-Mleux B, Jones BE, et al. Orexins/hypocretins excite basal forebrain cholinergic neurones. Neuroscience. 2001;108(2):177–81.
Fadel J, Burk JA. Orexin/hypocretin modulation of the basal forebrain cholinergic system: role in attention. Brain Res. 2010;1314:112–23.
Ishibashi M, Gumenchuk I, Kang B, Steger C, Lynn E, Molina NE, et al. Orexin receptor activation generates gamma band input to cholinergic and serotonergic arousal system neurons and drives an intrinsic Ca(2+)-dependent resonance in LDT and PPT cholinergic neurons. Front Neurol. 2015;6:120.
Irmak SO, de Lecea L. Basal forebrain cholinergic modulation of sleep transitions. Sleep. 2014;37(12):1941–51.
Boschen KE, Fadel JR, Burk JA. Systemic and intrabasalis administration of the orexin-1 receptor antagonist, SB-334867, disrupts attentional performance in rats. Psychopharmacology. 2009;206(2):205–13.
Piantadosi PT, Holmes A, Roberts BM, Bailey AM. Orexin receptor activity in the basal forebrain alters performance on an olfactory discrimination task. Brain Res. 2015;1594:215–22.
Alam MN, Kumar S, Suntsova N, Bashir T, Szymusiak R, McGinty D. GABAergic regulation of the perifornical-lateral hypothalamic neurons during non-rapid eye movement sleep in rats. Neuroscience. 2010;167(3):920–8.
Vazquez-DeRose J, Schwartz MD, Nguyen AT, Warrier DR, Gulati S, Mathew TK et al. Hypocretin/orexin antagonism enhances sleep-related adenosine and GABA neurotransmission in rat basal forebrain. Brain Struct Funct. 2014
Avolio E, Alo R, Carelli A, Canonaco M. Amygdalar orexinergic-GABAergic interactions regulate anxiety behaviors of the Syrian golden hamster. Behav Brain Res. 2011;218(2):288–95.
Gottesmann C. GABA mechanisms and sleep. Neuroscience. 2002;111(2):231–9.
Harrison NL. Mechanisms of sleep induction by GABA(A) receptor agonists. J Clin Psychiatry. 2007;68 Suppl 5:6–12.
Matsuki T, Nomiyama M, Takahira H, Hirashima N, Kunita S, Takahashi S, et al. Selective loss of GABA(B) receptors in orexin-producing neurons results in disrupted sleep/wakefulness architecture. Proc Natl Acad Sci U S A. 2009;106(11):4459–64.
Apergis-Schoute J, Iordanidou P, Faure C, Jego S, Schone C, Aitta-Aho T, et al. Optogenetic evidence for inhibitory signaling from orexin to MCH neurons via local microcircuits. J Neurosci. 2015;35(14):5435–41.
Li Y, van den Pol AN. Direct and indirect inhibition by catecholamines of hypocretin/orexin neurons. J Neurosci. 2005;25(1):173–83.
Gotter AL, Garson SL, Stevens J, Munden RL, Fox SV, Tannenbaum PL, et al. Differential sleep-promoting effects of dual orexin receptor antagonists and GABAA receptor modulators. BMC Neurosci. 2014;15:109.
Ida T, Nakahara K, Murakami T, Hanada R, Nakazato M, Murakami N. Possible involvement of orexin in the stress reaction in rats. Biochem Biophys Res Commun. 2000;270(1):318–23.
Sakamoto F, Yamada S, Ueta Y. Centrally administered orexin-A activates corticotropin-releasing factor-containing neurons in the hypothalamic paraventricular nucleus and central amygdaloid nucleus of rats: possible involvement of central orexins on stress-activated central CRF neurons. Regul Pept. 2004;118(3):183–91.
Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M, Komuro I, et al. Attenuated defense response and low basal blood pressure in orexin knockout mice. Am J Physiol-Reg I. 2003;285(3):R581–93.
Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8(4):383–95.
Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, et al. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J Neurosci. 2004;24(50):11439–48.
Winsky-Sommerer R, Boutrel B, de Lecea L. Stress and arousal—the corticotrophin-releasing factor/hypocretin circuitry. Mol Neurobiol. 2005;32(3):285–94.
Bonnavion P, Jackson AC, Carter ME, de Lecea L. Antagonistic interplay between hypocretin and leptin in the lateral hypothalamus regulates stress responses. Nat Commun. 2015;6. This article reveals that Hcrt neurons regulate corticosterone release and peripheral metabolic signals modulate activity of Hcrt neurons in response to stress.
Quarta D, Smolders I. Rewarding, reinforcing and incentive salient events involve orexigenic hypothalamic neuropeptides regulating mesolimbic dopaminergic neurotransmission. Eur J Pharm Sci : Off J Eur Fed Pharm Sci. 2014;57:2–10.
van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci. 2004;7(5):493–4.
Horvath TL, Diano S, van den Pol AN. Synaptic interaction between hypocretin (orexin) and neuropeptide Y cells in the rodent and primate hypothalamus: a novel circuit implicated in metabolic and endocrine regulations. J Neurosci. 1999;19(3):1072–87.
Muroya S, Funahashi H, Yamanaka A, Kohno D, Uramura K, Nambu T, et al. Orexins (hypocretins) directly interact with neuropeptide Y, POMC and glucose-responsive neurons to regulate Ca 2+ signaling in a reciprocal manner to leptin: orexigenic neuronal pathways in the mediobasal hypothalamus. Eur J Neurosci. 2004;19(6):1524–34.
Fu LY, Acuna-Goycolea C, van den Pol AN. Neuropeptide Y inhibits hypocretin/orexin neurons by multiple presynaptic and postsynaptic mechanisms: tonic depression of the hypothalamic arousal system. J Neurosci. 2004;24(40):8741–51.
Horvath TL, Abizaid A, Dietrich MO, Li Y, Takahashi JS, Bass J. Ghrelin-immunopositive hypothalamic neurons tie the circadian clock and visual system to the lateral hypothalamic arousal center. Mol Metab. 2012;1(1–2):79–85.
Toshinai K, Date Y, Murakami N, Shimada M, Mondal MS, Shimbara T, et al. Ghrelin-induced food intake is mediated via the orexin pathway. Endocrinology. 2003;144(4):1506–12.
Li X, Marchant NJ, Shaham Y. Opposing roles of cotransmission of dynorphin and hypocretin on reward and motivation. Proc Natl Acad Sci U S A. 2014;111(16):5765–6.
Crocker A, Espana RA, Papadopoulou M, Saper CB, Faraco J, Sakurai T, et al. Concomitant loss of dynorphin, NARP, and orexin in narcolepsy. Neurology. 2005;65(8):1184–8.
Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450(7168):420–4.
Brisbare-Roch C, Dingemanse J, Koberstein R, Hoever P, Aissaoui H, Flores S, et al. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med. 2007;13(2):150–5.
Vassalli A, Dellepiane JM, Emmenegger Y, Jimenez S, Vandi S, Plazzi G, et al. Electroencephalogram paroxysmal theta characterizes cataplexy in mice and children. Brain : J Neurol. 2013;136(Pt 5):1592–608.
Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30(2):345–54.
Liu M, Blanco-Centurion C, Konadhode R, Begum S, Pelluru D, Gerashchenko D, et al. Orexin gene transfer into zona incerta neurons suppresses muscle paralysis in narcoleptic mice. J Neurosci. 2011;31(16):6028–40.
Mieda M, Hasegawa E, Kisanuki YY, Sinton CM, Yanagisawa M, Sakurai T. Differential roles of orexin receptor-1 and -2 in the regulation of non-REM and REM sleep. J Neurosci. 2011;31(17):6518–26.
Burgess CR, Oishi Y, Mochizuki T, Peever JH, Scammell TE. Amygdala lesions reduce cataplexy in orexin knock-out mice. J Neurosci. 2013;33(23):9734–42.
Elbaz I, Yelin-Bekerman L, Nicenboim J, Vatine G, Appelbaum L. Genetic ablation of hypocretin neurons alters behavioral state transitions in zebrafish. J Neurosci. 2012;32(37):12961–72.
Prober DA, Rihel J, Onah AA, Sung RJ, Schier AF. Hypocretin/orexin overexpression induces an insomnia-like phenotype in zebrafish. J Neurosci. 2006;26(51):13400–10.
Mignot E, Lammers GJ, Ripley B, Okun M, Nevsimalova S, Overeem S, et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59(10):1553–62.
Baier PC, Hallschmid M, Seeck-Hirschner M, Weinhold SL, Burkert S, Diessner N, et al. Effects of intranasal hypocretin-1 (orexin A) on sleep in narcolepsy with cataplexy. Sleep Med. 2011;12(10):941–6.
Dubey AK, Handu SS, Mediratta PK. Suvorexant: the first orexin receptor antagonist to treat insomnia. J Pharmacol Pharmacother. 2015;6(2):118–21.
Michelson D, Snyder E, Paradis E, Chengan-Liu M, Snavely DB, Hutzelmann J, et al. Safety and efficacy of suvorexant during 1-year treatment of insomnia with subsequent abrupt treatment discontinuation: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2014;13(5):461–71. In this clinical study, suvorexant, a potent and selective Hcrt receptor antagonist, was shown to be effective, safe and well tolerated for treating insomnia.
Hoyer D, Durst T, Fendt M, Jacobson LH, Betschart C, Hintermann S, et al. Distinct effects of IPSU and suvorexant on mouse sleep architecture. Front Neurosci. 2013;7:235.
Han F, Lin L, Warby SC, Faraco J, Li J, Dong SX, et al. Narcolepsy onset is seasonal and increased following the 2009 H1N1 pandemic in China. Ann Neurol. 2011;70(3):410–7.
Heier MS, Gautvik KM, Wannag E, Bronder KH, Midtlyng E, Kamaleri Y, et al. Incidence of narcolepsy in Norwegian children and adolescents after vaccination against H1N1 influenza A. Sleep Med. 2013;14(9):867–71.
Kornum BR, Faraco J, Mignot E. Narcolepsy with hypocretin/orexin deficiency, infections and autoimmunity of the brain. Curr Opin Neurobiol. 2011;21(6):897–903.
Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, et al. A key role for orexin in panic anxiety. Nat Med. 2010;16(1):111–5.
Dauvilliers Y, Bayard S, Shneerson JM, Plazzi G, Myers AJ, Garcia-Borreguero D. High pain frequency in narcolepsy with cataplexy. Sleep Med. 2011;12(6):572–7.
Watanabe S, Kuwaki T, Yanagisawa M, Fukuda Y, Shimoyama M. Persistent pain and stress activate pain-inhibitory orexin pathways. Neuroreport. 2005;16(1):5–8.
Mobarakeh JI, Takahashi K, Sakurada S, Nishino S, Watanabe H, Kato M, et al. Enhanced antinociception by intracerebroventricularly and intrathecally-administered orexin A and B (hypocretin-1 and -2) in mice. Peptides. 2005;26(5):767–77.
Rainero I, Gallone S, Valfre W, Ferrero M, Angilella G, Rivoiro C, et al. A polymorphism of the hypocretin receptor 2 gene is associated with cluster headache. Neurology. 2004;63(7):1286–8.
Hara J, Yanagisawa Y, Sakurai T. Difference in obesity phenotype between orexin-knockout mice and orexin neuron-deficient mice with same genetic background and environmental conditions. Neurosci Lett. 2005;380(3):239–42.
Haynes AC, Jackson B, Chapman H, Tadayyon M, Johns A, Porter RA, et al. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul Pept. 2000;96(1–2):45–51.
Funato H, Tsai AL, Willie JT, Kisanuki Y, Williams SC, Sakurai T, et al. Enhanced orexin receptor-2 signaling prevents diet-induced obesity and improves leptin sensitivity. Cell Metab. 2009;9(1):64–76.
Burdakov D, Karnani MM, Gonzalez A. Lateral hypothalamus as a sensor-regulator in respiratory and metabolic control. Physiol Behav. 2013;121:117–24.
Goforth PB, Leinninger GM, Patterson CM, Satin LS, Myers MG. Leptin acts via lateral hypothalamic area neurotensin neurons to inhibit orexin neurons by multiple GABA-independent mechanisms. J Neurosci. 2014;34(34):11405–15.
Karnani MM, Apergis-Schoute J, Adamantidis A, Jensen LT, de Lecea L, Fugger L, et al. Activation of central orexin/hypocretin neurons by dietary amino acids. Neuron. 2011;72(4):616–29.
Tsujino N, Yamanaka A, Ichiki K, Muraki Y, Kilduff TS, Yagami K, et al. Cholecystokinin activates orexin/hypocretin neurons through the cholecystokinin A receptor. J Neurosci. 2005;25(32):7459–69.
Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci U S A. 2007;104(25):10685–90.
Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38(5):701–13.
Akiyama M, Yuasa T, Hayasaka N, Horikawa K, Sakurai T, Shibata S. Reduced food anticipatory activity in genetically orexin (hypocretin) neuron-ablated mice. Eur J Neurosci. 2004;20(11):3054–62.
Mieda M, Williams SC, Sinton CM, Richardson JA, Sakurai T, Yanagisawa M. Orexin neurons function in an efferent pathway of a food-entrainable circadian oscillator in eliciting food-anticipatory activity and wakefulness. J Neurosci. 2004;24(46):10493–501.
Gooley JJ, Schomer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci. 2006;9(3):398–407.
Mieda M, Williams SC, Richardson JA, Tanaka K, Yanagisawa M. The dorsomedial hypothalamic nucleus as a putative food-entrainable circadian pacemaker. Proc Natl Acad Sci U S A. 2006;103(32):12150–5.
Thorpe AJ, Kotz CM. Orexin A in the nucleus accumbens stimulates feeding and locomotor activity. Brain Res. 2005;1050(1–2):156–62.
Piccoli L, Micioni Di Bonaventura MV, Cifani C, Costantini VJ, Massagrande M, Montanari D, et al. Role of orexin-1 receptor mechanisms on compulsive food consumption in a model of binge eating in female rats. Neuropsychopharmacol : Off Publ Am Coll Neuropsychopharmacol. 2012;37(9):1999–2011.
Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289(23):3095–105.
Nollet M, Leman S. Role of orexin in the pathophysiology of depression: potential for pharmacological intervention. CNS Drugs. 2013;27(6):411–22.
Kim TK, Kim JE, Park JY, Lee JE, Choi J, Kim H, et al. Antidepressant effects of exercise are produced via suppression of hypocretin/orexin and melanin-concentrating hormone in the basolateral amygdala. Neurobiol Dis. 2015;79:59–69.
Nollet M, Gaillard P, Minier F, Tanti A, Belzung C, Leman S. Activation of orexin neurons in dorsomedial/perifornical hypothalamus and antidepressant reversal in a rodent model of depression. Neuropharmacology. 2011;61(1–2):336–46.
Jalewa J, Wong-Lin K, McGinnity TM, Prasad G, Holscher C. Increased number of orexin/hypocretin neurons with high and prolonged external stress-induced depression. Behav Brain Res. 2014;272:196–204.
Mikrouli E, Wortwein G, Soylu R, Mathe AA, Petersen A. Increased numbers of orexin/hypocretin neurons in a genetic rat depression model. Neuropeptides. 2011;45(6):401–6.
James MH, Campbell EJ, Walker FR, Smith DW, Richardson HN, Hodgson DM, et al. Exercise reverses the effects of early life stress on orexin cell reactivity in male but not female rats. Front Behav Neurosci. 2014;8:244.
Cho CH, Lee HJ, Woo HG, Choi JH, Greenwood TA, Kelsoe JR. CDH13 and HCRTR2 may be associated with hypersomnia symptom of bipolar depression: a genome-wide functional enrichment pathway analysis. Psychiatr Invest. 2015;12(3):402–7.
Schmidt FM, Arendt E, Steinmetzer A, Bruegel M, Kratzsch J, Strauss M, et al. CSF-hypocretin-1 levels in patients with major depressive disorder compared to healthy controls. Psychiatry Res. 2011;190(2–3):240–3.
Salomon RM, Ripley B, Kennedy JS, Johnson B, Schmidt D, Zeitzer JM, et al. Diurnal variation of cerebrospinal fluid hypocretin-1 (orexin-A) levels in control and depressed subjects. Biol Psychiatry. 2003;54(2):96–104.
Nocjar C, Zhang J, Feng P, Panksepp J. The social defeat animal model of depression shows diminished levels of orexin in mesocortical regions of the dopamine system, and of dynorphin and orexin in the hypothalamus. Neuroscience. 2012;218:138–53.
Scott MM, Marcus JN, Pettersen A, Birnbaum SG, Mochizuki T, Scammell TE, et al. Hcrtr1 and 2 signaling differentially regulates depression-like behaviors. Behav Brain Res. 2011;222(2):289–94.
Arendt DH, Ronan PJ, Oliver KD, Callahan LB, Summers TR, Summers CH. Depressive behavior and activation of the orexin/hypocretin system. Behav Neurosci. 2013;127(1):86–94.
Huang YS, Guilleminault C, Chen CH, Lai PC, Hwang FM. Narcolepsy-cataplexy and schizophrenia in adolescents. Sleep Med. 2014;15(1):15–22.
Lambe EK, Liu RJ, Aghajanian GK. Schizophrenia, hypocretin (orexin), and the thalamocortical activating system. Schizophr Bull. 2007;33(6):1284–90.
Chien YL, Liu CM, Shan JC, Lee HJ, Hsieh MH, Hwu HG, et al. Elevated plasma orexin A levels in a subgroup of patients with schizophrenia associated with fewer negative and disorganized symptoms. Psychoneuroendocrinology. 2015;53:1–9.
Borgland SL, Labouebe G. Orexin/hypocretin in psychiatric disorders: present state of knowledge and future potential. Neuropsychopharmacol : Off Publ Am Coll Neuropsychopharmacol. 2010;35(1):353–4.
Dalal MA, Schuld A, Pollmacher T. Lower CSF orexin A (hypocretin-1) levels in patients with schizophrenia treated with haloperidol compared to unmedicated subjects. Mol Psychiatry. 2003;8(10):836–7.
Annerbrink K, Westberg L, Olsson M, Andersch S, Sjodin I, Holm G, et al. Panic disorder is associated with the Val308Iso polymorphism in the hypocretin receptor gene. Psychiatr Genet. 2011;21(2):85–9.
Johnson PL, Samuels BC, Fitz SD, Federici LM, Hammes N, Early MC, et al. Orexin 1 receptors are a novel target to modulate panic responses and the panic brain network. Physiol Behav. 2012;107(5):733–42.
Fortuyn HA, Lappenschaar MA, Furer JW, Hodiamont PP, Rijnders CA, Renier WO, et al. Anxiety and mood disorders in narcolepsy: a case-control study. Gen Hosp Psychiatry. 2010;32(1):49–56.
Fronczek R, van Geest S, Frolich M, Overeem S, Roelandse FW, Lammers GJ, et al. Hypocretin (orexin) loss in Alzheimer’s disease. Neurobiol Aging. 2012;33(8):1642–50.
Kasanuki K, Iseki E, Kondo D, Fujishiro H, Minegishi M, Sato K, et al. Neuropathological investigation of hypocretin expression in brains of dementia with Lewy bodies. Neurosci Lett. 2014;569:68–73.
Wienecke M, Werth E, Poryazova R, Baumann-Vogel H, Bassetti CL, Weller M, et al. Progressive dopamine and hypocretin deficiencies in Parkinson’s disease: is there an impact on sleep and wakefulness? J Sleep Res. 2012;21(6):710–7.
Drouot X, Moutereau S, Lefaucheur JP, Palfi S, Covali-Noroc A, Margarit L, et al. Low level of ventricular CSF orexin-A is not associated with objective sleepiness in PD. Sleep Med. 2011;12(9):936–7.
Chang GQ, Karatayev O, Liang SC, Barson JR, Leibowitz SF. Prenatal ethanol exposure stimulates neurogenesis in hypothalamic and limbic peptide systems: possible mechanism for offspring ethanol overconsumption. Neuroscience. 2012;222:417–28.
Chang GQ, Karatayev O, Leibowitz SF. Prenatal exposure to nicotine stimulates neurogenesis of orexigenic peptide-expressing neurons in hypothalamus and amygdala. J Neurosci. 2013;33(34):13600–11.
Morgan AJ, Harrod SB, Lacy RT, Stanley EM, Fadel JR. Intravenous prenatal nicotine exposure increases orexin expression in the lateral hypothalamus and orexin innervation of the ventral tegmental area in adult male rats. Drug Alcohol Depend. 2013;132(3):562–70.
Braun SM, Jessberger S. Adult neurogenesis: mechanisms and functional significance. Development. 2014;141(10):1983–6.
Hsieh J. Orchestrating transcriptional control of adult neurogenesis. Genes Dev. 2012;26(10):1010–21.
Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, et al. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci U S A. 2001;98(8):4710–5.
Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70(4):687–702.
Mirescu C, Peters JD, Noiman L, Gould E. Sleep deprivation inhibits adult neurogenesis in the hippocampus by elevating glucocorticoids. Proc Natl Acad Sci U S A. 2006;103(50):19170–5.
Haan N, Goodman T, Najdi-Samiei A, Stratford CM, Rice R, El Agha E, et al. Fgf10-expressing tanycytes add new neurons to the appetite/energy-balance regulating centers of the postnatal and adult hypothalamus. J Neurosci. 2013;33(14):6170–80.
Lee DA, Bedont JL, Pak T, Wang H, Song J, Miranda-Angulo A, et al. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat Neurosci. 2012;15(5):700–2.
Lee DA, Yoo S, Pak T, Salvatierra J, Velarde E, Aja S et al. Dietary and sex-specific factors regulate hypothalamic neurogenesis in young adult mice. Frontiers in Neuroscience. 2014;8.
Pierce AA, Xu AW. De novo neurogenesis in adult hypothalamus as a compensatory mechanism to regulate energy balance. J Neurosci. 2010;30(2):723–30.
Sousa-Ferreira L, Alvaro AR, Aveleira C, Santana M, Brandao I, Kugler S et al. Proliferative hypothalamic neurospheres express NPY, AGRP, POMC, CART and orexin-A and differentiate to functional neurons. PloS one. 2011;6(5).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The de Lecea laboratory receives funding from Merck and Johnson & Johnson.
Shi-Bin Li, Jeff R. Jones, and Luis de Lecea declare that they have no other conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Sleep Disorders
Rights and permissions
About this article
Cite this article
Li, SB., Jones, J.R. & de Lecea, L. Hypocretins, Neural Systems, Physiology, and Psychiatric Disorders. Curr Psychiatry Rep 18, 7 (2016). https://doi.org/10.1007/s11920-015-0639-0
Published:
DOI: https://doi.org/10.1007/s11920-015-0639-0