Abstract
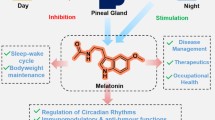
Melatonin is ubiquitous molecule with wide distribution in nature and is produced by many living organisms. In human beings, pineal gland is the major site for melatonin production and to lesser extent by retina, lymphocytes, bone marrow, gastrointestinal tract, and thymus. Melatonin as a neurohormone is released into circulation wherein it penetrates all tissues of the body. Melatonin synthesis and secretion is supressed by light and enhanced by dark. Melatonin mostly exerts its effect through different pathways with melatonin receptor 1 (MT1) and melatonin receptor 2 (MT2) being the predominant type of receptor that are mainly expressed by many mammalian organs. Melatonin helps to regulate sleep patterns and circadian rhythms. In addition, melatonin acts as an antioxidant and scavenges excessive free radicals generated in the body by anti-excitatory and anti-inflammatory properties. A multiple array of other functions are displayed by melatonin that include oncostatic, hypnotic, immune regulation, reproduction, puberty timing, mood disorders, and transplantation. Deficiencies in the production or synthesis of melatonin have been found to be associated with onset of many disorders like breast cancer and neurodegenerative disorders. Melatonin could be used as potential analgesic drug in diseases associated with pain and it has quite promising role there. In the past century, a growing interest has been developed regarding the wide use of melatonin in treating various diseases like inflammatory, gastrointestinal, cancer, mood disorders, and others. Several melatonin agonists have been synthesized and are widely used in disease treatment. In this review, an effort has been made to describe the biochemistry of melatonin along with its therapeutic potential in various diseases of humans.
Graphical Abstract

Similar content being viewed by others
Introduction
The word ‘melatonin,’ comes from a Greek word ‘melas meaning dark’ and ‘tonos meaning hormone of darkness’ is a ubiquitous hormone found in all living organisms of animal kingdom. Melatonin (N-acetyl-5-methoxytryptamine), an indoleamine, is predominantly secreted and synthesized by the pineal gland through hydroxylation of essential amino acid i.e., tryptophan at carbon 5 forming 5-hydroxytryptophan by tryptophan hydroxylase. Decarboxylation of 5-hydroxytryptophan yields a key neurotransmitter, 5-hydroxytryptamine (serotonin) which on acetylation by enzyme arylalkylamine N-acetyltransferase produces N-acetyl serotonin, the immediate precursor of melatonin. Subsequently, the enzyme acetyl-serotonin-methyltransferase (ASMT) through the process of methylation converts N-acetyl serotonin to melatonin (Coon et al. 2001) (Fig. 1). Pineal gland is the principal synthesizer and secretor of melatonin, in addition to many reports indicating the formation of melatonin in very small quantities by other organs namely retina, harderian gland (Cardinali and Rosner 1971), gastrointestinal tract (Bubenik 2002), and lymphocytes. The traces of melatonin have been significantly identified in different parts of higher plants like fruits, seeds, and leaves (Tan et al. 2016; Reiter et al. 2014). However, the levels in these plant parts are too low for supply to human beings. The wide distribution of melatonin in primitive bacteria depicts ancient origin of the molecule and has been retained from evolution of living creatures (Pshenichnyuk et al. 2017). In bacteria, melatonin has evolved by process of endosymbiosis. Earlier, melatonin was thought to be involved in the photosynthesis and metabolic pathways and free radical detoxification processes (Manchester et al. 2015; Tan et al. 2015). However, during the course of evolution, melatonin diversified and attained a pleiotropic nature showing predominant role not only in resisting oxidative stress but also affecting biological rhythms and reducing inflammatory states (Tan et al. 2010; Tamtaji et al. 2018). Different species have evolved divergent melatonin biosynthetic routes and the genes encoding the enzymes involved in these pathways (Back et al. 2016). The role of melatonin is quite evident from its involvement in multiple biosynthetic metabolic pathways. In unicellular and multicellular organisms, the enzymes involved in biosynthesis of melatonin located in subcellular localization may somehow have been changed (Lee et al. 2017). The separate subcellular sites of localization for biosynthesis of melatonin may be beneficial for its efficient control (Back et al. 2016; Byeon et al. 2015). The secretion of the melatonin is controlled by enzyme hydroxyindole-O-methyltransferase (HIOMT), also referred as acetyl-serotonin-methyltransferase (ASMT) which indirectly is commanded by photoneural system (Jang et al. 2010). Within the parenchymal cells, the presence of many capillaries permit excessive metabolic activity. Because the pineal lacks a blood–brain barrier, it shows resemblance to other periventricular glands like subfornical organ (SFO), median eminence, and subcommissural organ (SCO), which all are produced from ependymal cells in the third ventricle’s roof (Hissa et al. 2008). In living species, a circadian manner of melatonin secretion is observed with highest levels at night with 30–70-fold increase in N-acetyltransferase (NAT) enzyme activity. Melatonin production and release peak during the dark hours and fall during the day in all species studied (Binkley et al. 1988; Bolliet et al. 1996). The pineal gland does not store melatonin; however, the gland capacity of synthesis is reflected by its plasma concentration. The pineal gland responds directly to light in early non-mammalian vertebrates, whereas in higher vertebrates, it is no longer sensitive to light. Due to high lipid and water solubility of melatonin, its distribution is facilitated through most cell membranes including the blood–brain barrier (Pardridge and Mietus 1980). When released into circulation, it easily gains entry into different body fluids, cellular compartments, and tissues. Melatonin is mostly synthesized at night with its peak plasma concentration found around 3:00 to 4:00 a.m. Mainly, melatonin in blood is largely bound to albumin (70%) and to orosomucoid or alpha-1-acid glycoprotein to lower extent. The melatonin in circulation can travel to all tissues in the body and easily modulate activity of brain by crossing blood–brain barrier. No or very less melatonin secretion occurs upto 3 months of age. Then, at the age of 3–4 years, the production of melatonin reaches its peak, and by the adult stage, it progressively drops down by 80%. Temporarily, this change has been associated with sexual maturity and not with growing size of body; however, production of constant melatonin during childhood is due to increasing body size (Waldhauser et al. 1993).
Factors Affecting Melatonin Synthesis
In humans, melatonin synthesis and generation is influenced by many factors as represented in Table 1 (Simonneaux and Ribelayga 2003; Zawilska et al. 2009).
Edible Sources of Melatonin
Melatonin has been notably found in a variety of food items, whether edible plants or plant-based products. The plants and few food entities not only possess melatonin but also its precursor. In plants, melatonin presence is universal; however, the concentrations may vary from picograms to micrograms in plant tissue (Tan et al. 2012). Table 2 shows the amount of melatonin that has been detected by chromatographic and immunological techniques in food and plants. Melatonin in plants plays an important role in reducing oxidative stress, promotes growth and germination of seeds, improves resistance to stress, stimulates immune system, modulates circadian rhythms, controls closure of stomata on leaves, and antistress agent against drought, toxic chemicals, salinity, heavy metal stress, UV radiation, high and low ambient temperatures, water stress, and light-induced stress. Moreover, melatonin also shows it role in combating biotic stress in plants that includes various properties like antibacterial, antiviral, and antifungal effects.
Receptors and Mechanism of Action
Melatonin is a multifaceted hormone exhibiting its effects through endocrine, autocrine, and paracrine modes (Reiter 2003). Its action is facilitated either by receptor binding or acting directly. Within species, the melatonin receptors express considerable variability in density and location (Morgan et al. 1994; Liu et al. 2016). In mammals, melatonin shows its effect by binding to plasma membrane receptors, intracellular proteins like calmodulin or orphan nuclear receptors.
Melatonin also shows interaction with intracellular protein molecules such as calreticulin, calmodulin, and tubulin (Bolliet et al. 1996). Calmodulin is an intracellular secondary messenger. Melatonin directly competes with calcium for binding to calmodulin (Bolliet et al. 1996; Ekmekcioglu 2006) which may also be responsible for anti-proliferative effect observed in cancers. The immunomodulatory effects of melatonin are due to synthesis of IL-2 and IL-6 by mononuclear cells by binding of melatonin to Retinoid-related Orphan nuclear hormone receptor family (RZR/ROR) (Ekmekcioglu 2006).
In animal cells, melatonin mostly exerts its effects through membrane-bound G-protein coupled receptors (GPCR) (Jockers et al. 2016). In mammals, melatonin three designated GPCR are MT1, MT2, MT3 and also one nuclear receptors have been identified (Reppert et al. 1995; Nosjean et al. 2000). The receptors of melatonin are widely distributed and are found in brain, cardiovascular system, aorta, cardiac ventricular wall, cerebral and coronary arteries, gallbladder, liver, retina, parotid gland, appendix, cecum, colon, skin, pancreas, platelets, immune system cells, kidney, brown and white adipocytes, breast and ovarian granulosa cells, epithelial cells of prostrate, fetal kidney, and placental myometrium (Uz et al. 2005, Hardeland 2012). In the jejunal and colonic mucosa of the gastrointestinal tract, most of the receptors of melatonin are typically located (Bolliet et al. 1996). Since the melatonin exhibits its action through the involvement of various molecular pathways, the most described pathways involve activation of specific membrane receptors namely ML1 i.e., high affinity and low affinity i.e., ML2 sites (Dubocovich 1995; Morgan et al. 1994). ML1 acts directly on the target cells or via G-protein coupled receptors and contains two sub types MT1 and MT2 (Li et al. 2013), whereas the newly purified ML2 receptors also referred as MT3 protein that belongs to quinone reductases family (Cardinali et al. 1997). On the basis of chromosome location and molecular structure, the receptors of melatonin (MT1 and MT2) are described as distinct subtypes (Reppert et al. 1995; Dubocovich et al. 2003).
MT1 Receptor
MT1 also known as Mell a receptor is made up of 351 amino acids and is encoded by chromosome 4 in humans (Li et al. 2013). Mell a has a wide distribution in pars tuberalis and suprachiasmatic nucleus (SCN) of the hypothalamus the anatomical site of the circadian clock, cortex, thalamus, substantia nigra, cerebellum, nucleus accumbens, and retina (Jockers et al. 2008). The binding of melatonin to these high affinity receptors of GPCR super family in target cells causes inhibition of the adenylate cyclase/cAMP activity and increases phospholipase C/IP3 action (Ebisawa et al. 1994). MT1 receptors have two subgroups, MT1a and MT2b (Morgan et al. 1994). In cardiac vessels and SCN that express MT1 receptor helps to modulate circadian rhythms (Dubocovich et al. 1998; Liu et al. 1997) and constrict cardiac vessels (Doolen et al. 1998). Apart from these areas, other parts of the brain and peripheral tissues express MT1 (Clemens et al. 2001; Ram et al. 2002).
MT2 Receptor
It has 363 amino acids and is coded on human chromosome 11 (Li et al. 2013). MT2 receptors, also known as Mell b receptors, have low affinity and are involved in phosphoinositol hydrolysis (Brzezinski 1997), retinal physiology (Klein 1985), circadian rhythm modulation (Dubocovich et al. 1998), cardiac vessel dilation (Doolen et al. 1998), and inflammatory responses (Lotufo et al. 2001; Li and Witt-Enderby 2000; Masana and Dubocovich 2001; Von Gall et al. 2002). The localization of MT2 receptors is restricted as compared to MT1 and primarily they are found in retina and secondarily in hippocampus, cortex, paraventricular nucleus, and cerebellum (Zawilska et al. 2009; Li and Witt-Enderby 2000; Masana and Dubocovich 2001; Von Gall et al. 2002).
MT3 Receptor
Initially, MT3 was purified from the kidney of Syrian hamster and showed similar binding profile to MT2 (Dubocovich 1995; Molinari et al. 1996; Nosjean et al. 2000). It was discovered that the MT3 protein is 95% identical to human quinone reductase 2, a detoxifying enzyme (Nosjean et al. 2000). Leukotriene B4-induced leukocyte adherence is inhibited and intraocular pressure is reduced when MT3 receptors are activated (Dubocovich et al. 2003).
In addition to this, few properties of melatonin cannot be described by membrane receptors. Another group of receptors known as orphan nuclear hormone receptor superfamily RZR/ROR appears to be the natural ligand for melatonin. The immunomodulatory properties of melatonin are because of these nuclear bound receptors (Carrillo-Vico et al. 2003).
Melatonin Actions (Non-receptor Mediated)
Most of the activities of the melatonin are receptor mediated; however, a few occur with the involvement of receptor molecule and thus are non-receptor mediated and the prominent example is the free radical scavenging activity. Melatonin being a strong antioxidant is directly responsible for scavenging of the free radicals and so are its metabolites (Galano et al. 2013). It also activates many scavenging pathways and enhances the antioxidant enzyme activity (Barlow-Walden et al. 1995; Rodriguez et al. 2004). It also binds to the transition metals thus preventing the formation of hydroxyl radicals (Galano et al. 2015). Melatonin being highly concentrated in mitochondria protects proteins, lipids, and DNA from oxidative damage caused by free radicals (Venegas et al. 2012; Garcia et al. 2014). The antioxidant role of melatonin is of paramount importance for mitochondrial activities where free radical production is a natural phenomenon because of cellular respiration (Reiter et al. 2017). Apart from antioxidant role, melatonin plays a prime role not only in regulating respiratory chain complexes I and IV but also prevents the mutation and deletion of mitochondrial DNA (Jou et al. 2002). This action of melatonin results by direct interaction between melatonin and protein. Melatonin plays an antagonist role in protein degradation by its direct interaction with Ca2+-calmodulin thereby inhibiting the Ca2+/calmodulin-dependant protein kinase II activity and autophosphorylation (Benitrez-King et al. 1996). It prevents DNA damage by down regulation of the expression of ATM (a phosphoinositide 3-kinase-related kinase) and histone H2AX phosphorylation process involved in DNA degradation (Majidinia et al. 2017).
Melatonin and Brain
Melatonin has a diverse role and mostly affects brain. It has role in regulating circadian rhythm, seasonal adaptation, and puberty development pubertal development (Pandi et al. 2008). Melatonin is associated with memory by regulating memory formation by directly affecting hippocampal neurons (Comai and Gobbi 2014). It also controls posture and balance of the body (Pandi et al. 2008). Melatonin has antinociceptive, antidepressant, anxiolytic, and locomotor regulating effects (Uz et al. 2005). Melatonin has neuroprotective, lowering blood pressure, modulating pain, vascular, retinal, osteoblast differentiation, seasonal reproductive, ovarian physiology, anti-tumor, and antioxidant properties (Li et al. 2013). From hypothalamic neurons, secretion of gonadotrophin-releasing hormone (GnRH) is regulated by melatonin that further affects synthesis of follicle stimulating hormone (FSH) and luteinizing hormone (LH) (Dubocovich et al. 2003). In granulosa cells, the production of progesterone is promoted by melatonin (Dubocovich et al. 2003). Melatonin also inhibits estrogen receptor expression and estrogen activation (Carlberg 2000). Melatonin has been shown very helpful in treating neurological disorders such as Parkinsonism (Gunata et al. 2020), Alzheimer’s disease (Vecchierini et al. 2021), brain edema and traumatic brain injury (Dehghan et al. 2013), depression (Grima et al. 2018), cerebral ischemia (Tang et al. 2014), hyperhomocysteinuria (Karolczak and Watala 2021), glioma (Lai et al. 2019), and phenylketonuria (Yano et al. 2016). Melatonin was shown to inhibit amyloidosis (Shukla et al. 2017).
Antioxidant, Anti-inflammatory, and Neuroprotective Role of Melatonin
In the humans, there is a high consumption of oxygen in the brain i.e., 20% and this increased consumption causes oxidative stress and generates toxic free radical molecules in the body. These high reactive molecules damage DNA, proteins, and cell membrane (Gupta et al. 2003). The presence of considerable amount fat in membrane and myelin sheaths enhances the damage by free radicals thus creating an imbalance between oxidants and antioxidants (Skaper et al. 1999). The damage by reactive oxygen species (ROS) results in compromised blood–brain barrier and enhanced expression of excitatory neurotransmitter glutamate to extracellular space thereby triggers the depolarization (Gilman et al. 1993). The ROS also results in the alteration of gene expression, initiate apoptotic cascade, and decreased neuron viability (Gilgun-Sherki et al. 2002). Melatonin scavenges free radicals endogenously through its role as an antioxidant (Tordjman et al. 2017). The intake of melatonin supplementation enhances the superoxide dismutase (SOD) and glutathione peroxidase (GPx) activity (Mayo et al. 2002). Thus, melatonin exerts its neuroprotective potential through its antioxidant power. The onset of stroke results in the massive destruction of cells with enhanced production of ROS and inflammation. The neuron survival is dependent on active energy metabolism hence, any obstruction in the cerebral flow of blood, restricted glucose and oxygen supply results in ischemic stroke that can have catastrophic implication on neurons (Flynn et al. 2008). In case of glucose and oxygen scarcity, the cell survival is compromised through many pathways. In particular, the function of ATP-dependent Na+/K+-ATPase is compromised which results in the intracellular accumulation of Na+ resulting in anoxic depolarization and activation of voltage-gated calcium channels and reduction in Na2+/Ca2+ exchange, the disturbance results in the intracellular accumulation of Ca2+ which initiates cell injury (Stys 1998). Melatonin a potential antioxidant protects from ischemic injury (Watson et al. 2016; Wu et al. 2017).
The administration of melatonin in animal models with induced stroke results in the reduction of cerebral infarction (Sinha et al. 2001; Pei et al. 2003). Melatonin imparts a protective ability in gray and white matter, diminishes the inflammatory cascade and permeability of blood–brain (Chen et al. 2006; Lee et al. 2007). Melatonin injection attenuates oxidative brain injury in rat models (Ersahin et al. 2009; Wu et al. 2017). Melatonin also helps in Ca2+ homeostasis and reduction in extracellular glutamate levels by preventing its release into oxygen glucose-deprived rat model of ischemia (Patiño et al. 2016). In rat models with induced injury to brain, melatonin showed anti-inflammatory properties as it reduces the movement of macrophages/monocytes and neutrophils in circulation to the damaged area (Lee et al. 2007). Paredes et al. (2015) reported that intake of melatonin resulted in the significant decrease of intereukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), BAD, and BAX in the ischemic area of both hippocampus and cortex compared to non-administration group.
Alzheimer’s disease (AD) is an age-related disorder that results by the deposition of toxic proteins β-amyloid (Aβ) and neurofibrillary tangles (NFTs) in memory-related areas resulting in progressive cognitive behavior decline (Ittner and Gotz 2011; He et al. 2010). Neuronal loss and oxidative stress are triggered by the deposition of harmful protein molecules (Sultana and Butterfield 2010; Jeong 2017). Oxidative stress resulting due to accumulation of Aβ generated free radicals, membrane dysfunction, and inflammation and thereby plays a critical role in the onset of AD (Prasad 2017; Nesi et al. 2017). A recent study has reported antiamyloidogenic melatonin action on AD (Shukla et al. 2017). Melatonin also halts the synthesis of amyloid precursor protein (APP) which inturn disrupts the Aβ formation (Lahiri 1999). The long-time administration of melatonin is known to prevent the accumulation of Aβ in hippocampus and cortex in transgenic mice (Olcese et al. 2009). In AD, melatonin acts as a strong antioxidant as it diminishes Aβ-facilitated oxidative stress and lipid peroxidation (Daniels et al. 1998; Shukla et al. 2017). It has been reported that melatonin maintains the level of certain antioxidants like catalase, GPx, and SOD in the cortex of AD transgenic mice (Olcese et al. 2009).
Millions of people are suffering from Parkinson’s disease (PD) worldwide (Elbaz and Moisan 2008; Wirdefeldt et al. 2011). PD is a neurodegenerative disease with multiple etiological factors associated with the onset of PD such as genetics, age, smoking, dairy products consumption, and exposure to manganese and lead (Elbaz and Moisan 2008; Hughes et al. 2017; Ma et al. 2017). In substantia nigra pars compacta (SNC), there is dopaminergic neuronal loss in PD, thus resulting in striatal dopamine depletion which ultimately causes disturbance of the smooth coordinated muscle resulting in rigidity, tremor, bradykinesia, and postural problems (Tansey et al. 2007; Maguire-Zeiss and Federoff 2010). A number of reports have demonstrated the age-related PD is accompanied with oxidative pressure (Padurariu et al. 2013). In the onset of PD, free radicals are the initiating factors. The integral features of this disease are insomnia and depression in PD, and disturbances in sleep are related to psychiatric signs and decline in cognitive feature. The various factors that cause PD are shown in Fig. 2.
In the striatum and hippocampus, melatonin administration prevents peroxidation of lipids thereby arresting the neuronal death in an MPTP-induced PD model (Antolin et al. 2002). In animal models induced with PD, melatonin regulates the antioxidant enzyme activity of SOD and catalase in the nigrostriatal pathway in 6-OHDA. Therefore, it can be seen that melatonin exhibits its neuroprotective properties through its antioxidant and anti-inflammatory actions.
Melatonin and Hypertension
Hypertension is more frequently observed in obese individuals in comparison to lean cases, and melatonin’s role in modulating and regulating blood pressure seems an interesting subject for researchers from many years (Poirier et al. 2006; Qin et al. 2013; Cook et al. 2011). In mammals, melatonin regulates heart rate and arterial blood pressure (BP) (Simko and Pechanova 2009; Reiter et al. 2010) as the receptors for melatonin are identified in heart and different arterial beds (Capsoni et al. 1994; Krause et al. 1995; Pang et al. 1993, 1996). Subjects with hypertension exhibit disturbed day–night rhythms with changes in sympathetic and parasympathetic cardiac tone (Nakano et al. 2001). Individuals suffering from coronary heart ailment an outcome of the hypertension show reduced melatonin levels during the night hours (Brugger et al. 1995). Similarly, pinealectomy in rats has been reported to result in hypertension, and administration of endogenous melatonin has inhibited the increase in BP in pinealectomised rats (Simko and Paulis 2007). There is strong evidence that people with hypertension during day hours have disordered circadian rhythms (Simko and Paulis 2007). From pineal gland, the melatonin secretion is controlled by SCN (Buijs et al. 2003), melatonin through its high affinity receptors sends feed back to SCN there by controls its own production and other circadian rhythms (Amaral and Cipolla-Neto 2018). Night hour’s melatonin secretion directly boosts circadian rhythms through the principal pacemaker and has a critical role in improving the day–night rhythms (Cipolla-Neto and Amaral 2018) and BP (Scheer et al. 2004). Grossman et al. (2006) reported that administration of melatonin (2 mg for 4 weeks) at bed time reduced the nocturnal systolic and diastolic BP. There are a number of ways by which melatonin exerts its influence on BP. ROS and reactive nitrogen species (RNS) have significant role in the occurrence of hypertension (Anwar et al. 2001; Pechanova et al. 2006), while antioxidants diminish the hypertensive effects of ROS and NOS. The melatonin is reported to reduce BP by lowering the intracellular superoxide anion content, malondialdehyde, NF-κB expression, and enhancing GPx activity (Girouard et al. 2004; Nava et al. 2003; Paulis 2006). In young women patients with hypertension, lower levels of nocturnal melatonin were considered as risk factor in hypertension development (Forman et al. 2010). In hypertensive patients, impairment in secretion of melatonin resulted in decrease of nocturnal levels of melatonin (Zeman et al. 2005). As a result of these findings, melatonin is a potent therapeutic molecule in non-dipper patients or cases with hypertensive heart disease or nocturnal hypertension (Reiter et al. 2010). The intake of melatonin in individuals with nocturnal hypertension reduced systolic and diastolic blood pressure (Grossman et al. 2011).
Melatonin and Diabetes
Diabetes mellitus (DM) is a disorder of carbohydrate metabolism that is associated with elevated glucose levels in blood and defects in secretion and action of insulin (Ali et al. 2017a, b; Bhat et al. 2017). Melatonin has gained its importance because of its role in sleep and circadian regulation. However, during recent past, it has gained importance for its role in glucose tolerance and type 2 diabetes (T2D) risk or treatment. This is due to partial discovery of T2D risk variants in MTNR1B and partially negative impact of disturbed circadian rhythms on glucose metabolism (Mason et al. 2020). The disturbance in circadian rhythm has been reported to result in metabolic syndrome including obesity and diabetes (Pulimeno et al. 2013). There are many reports about the role of melatonin in insulin secretion and glucose homeostasis. A diminished level of melatonin has been reported in patients with T2D (Prokopenko et al. 2009). A study conducted on the role of melatonin glucose homeostasis in young Zucker diabetic fatty (ZDF) rats, an experiment model of metabolic syndrome and T2D revealed that oral administration of melatonin results in anti-hyperglycemic effect in young ZDF rats through improvement in β-cell function (Agil et al. 2012). Studies have revealed that deficiency of melatonin receptor has a direct bearing on levels of pancreatic islet hormones and glucose transporters (Glut 1 and Glut 2) (Bazwinsky-Wutschke et al. 2014). The relation between melatonin and T2D on the basis of a finding that secretion of insulin is inversely proportional to concentration of melatonin in plasma (Peschke et al. 2013). Subduing the secretion of melatonin as a result of nocturnal light exposure could be vital parameter in onset of T2D (Fonken and Nelson 2014). A large number of literature suggest a correlation between disturbed sleep orders and decreased glucose tolerance and T2D (Donga et al. 2010; Yaggi et al. 2006). A study reported by Hajam et al. (2022) has reported the therapeutic efficacy of coadministration of insulin and melatonin rat models with diabetes-induced renal injury. The outcome of the study reported has revealed that the combination of insulin and melatonin may be quite effective to treat renal alterations caused by diabetes as confirmed by sera biochemical parameters, anti-inflammatory molecules in serum, and histoarchitecture changes in kidney (Hajam et al. 2022). The melatonin administration modulates the synthesis of insulin in the pancreatic β-cells as well as enhancing the optimistic insulin effect (Hajam and Rai 2019).
Melatonin and Cancer
During the last century, different reports have assessed the oncostatic properties of melatonin against various malignancies like colorectal, breast cancer, prostate cancer, leukemia, pancreatic cancer, and melanoma (Foon 1989). These studies provided a promising result in the breast cancer cells that express estrogen receptors. Cancer accounts for the most number of deaths globally after cardiovascular diseases (Ferlay et al. 2012; Fitzmaurice et al. 2015). Statistically lung cancer results in most number of deaths in both sexes, while prostate cancer figures top in male deaths and breast cancer among female deaths (Ferlay et al. 2012; Fan et al. 2015; Torre et al. 2016; James et al. 2017). The availability of data of neoplastic diseases upto now clearly suggests the progression of human cancers not only depends disease biological characteristics like mutation, overexpression of genes, grading, histology, but also on immunobiological response of patient that includes immune and endocrine system status as well (Foon 1989). Similarly, the malfunctioning of the immune system does not rely on immune cells activity but also on modulation of neuroendocrine physiology, that is primarily influenced by pineal system. The pineal gland exerts anti-tumor anti-proliferative functions through secretion of peptide hormones and various anticancer indole molecules and mostly widely the melatonin hormone (Brzezinski 1997). Prior to the characterization of pineal gland hormones, it was known that the onset of tumor and dissemination was due to pinealectomy (Buswell 1975). The associations between melatonin and cancer have been studied from many decades and a large number of epidemiological studies favor the protective potential of melatonin against cancer (Nooshinfar et al. 2017; Li et al. 2017). Different research studies have reported other than melatonin, many anticancer hormones may be produced by pineal gland (Anisomov et al. 2001). Various studies have demonstrated the preventive role of melatonin in different type of cancers (Cos and Sanchez-Barcelo 2000; Wang et al. 2012; Kanishi et al. 2000; Subramanian et al. 2007). An important property of melatonin that makes it helpful in combating the tumor is its ability to diminish neoplastic amplification with cytostatic and cytotoxic actions (Martin et al. 2007; Liu et al. 2016). Several other studies reported the protective role of melatonin against mammary cancer (Kosar et al. 2016; Gatti et al. 2017). A decrease in circulating melatonin has been reported to be associated with increased prevalence of mammary tumors through carcinogen 7,12-dimethylbenz(a)-anthracene (DMBA) the treatment with melatonin minimizes the incidence (Chu et al. 2018). Melatonin in combination with retinoic acid on MCF-7 hormone-dependant breast cancer cells has completely blocked the cell growth and reduced the cell number by activation of apoptosis (Margheri et al. 2012). Other researchers have reported that melatonin suppresses the progress of tumor through its ability to inhibit the differentiation of cell cycle (Sánchez-Sánchez et al. 2011; Cabrera et al. 2010; Martin et al. 2007).
Melatonin and Obesity
There is substantial evidence connecting impaired circadian clock and obesity development. Despite the fact that the causal association between obesity and chronodisruption is bi-directional (Bray and Young 2012), melatonin and its agonists administration have proven to be effective in circadian rhythm resetting (Zawilska et al. 2009) and correcting disorders associated to obesity (Cardinali et al. 2011; She et al. 2009; Oxenkrug and Summergrad 2010). Furthermore, obesity is linked to a variety of comorbidities like sleeping disorders and melatonin or other medications have shown to be effective (Cardinali et al. 2011). Melatonin is hypothesized to play a role in energy metabolism and body weight management. In the body of seasonal animals, melatonin’s involvement in modulating metabolism and fat mass was first studied and established (Bartness and Wade 1985), and it was connected to its activity as a seasonal and circadian rhythm regulator (Arendt 2006). Depending on the animal species, any rise in circulation melatonin levels owing to photoperiodic variations or exogenous melatonin injection was eventually related with a loss or increase in body fat mass in these seasonal animals (Bartness and Wade 1985). Exposure to long photoperiod conditions of obese Zucker rats resulted in increased body weight as compared to short photoperiod exposed animals (Larkin et al. 1991). In accord, the elimination of the pineal gland decreased levels of melatonin in circulation and in obese rats elevated body weight as compared to healthy rats after 3 weeks (Prunet-Marcassus et al. 2003). In normal rats when postoperative duration was increased to 2 months, they gained weight in both the body and the heart (Kurcer et al. 2006). Melatonin (30 mg/kg/day, i.p. 1 h before lights-out) could prevent these pinealectomy-induced changes for 3 weeks (Prunet-Marcassus et al. 2003). The same researchers found that melatonin given in the same way could minimize body weight gain caused by a high-fat meal while having no effect on overall food intake (Prunet-Marcassus et al. 2003). Obesity-induced dyslipidemia symptoms have been demonstrated to be improved by melatonin. This was first noticed in non-obese hypercholesterolemia rats (Hussain 2007), and later validated in different obesity-induced rat models (Agil et al. 2012). Melatonin performs its diverse functions via receptor-mediated or non-receptor-mediated routes, as previously indicated (Venegas et al. 2012). The therapeutic potency of melatonin in treatment and prevention of obesity has been well summarized with high efficacy results in animal models (Barrenetxe et al. 2004; Shieh et al. 2009).
Melatonin and Autoimmunity
Multiple studies have linked onset of immune-compromised diseases like rheumatoid arthritis (RA), multiple sclerosis (MS), systemic lupus erythematosus (SLE) with exogenous and endogenous production of melatonin despite the scarcity of data on the relationship of melatonin to other autoimmune diseases. In this regard, psoriatic patients have shown to have disturbances in secretion of circadian melatonin (Mozzanica et al. 1988). In individuals with autoimmune hearing loss, melatonin inhibits proliferation of lymphocytes induced by type II collagen (Lopez-Gonzalez et al. 1997) and protects against idiopathic membranous nephropathy in an experimental model (Wu et al. 2012). Globally, 1% of the population is affected by Rheumatoid arthritis (RA) and the effects of melatonin on this autoimmune disorder appear to be debatable (McInnes and Schett 2011). RA is a progressively inflammatory disease that severely affects joints thereby leading to severe disability (Ali et al. 2017a, b). Several studies using experimental arthritis models suggest melatonin (endogenous and exogenous) has a negative effect. As a result, animals kept in constant darkness develop severe form of collagen-induced arthritis with increased titers of sera anti-collagen antibodies in comparison to animals living in constant light. Pinealectomy was used to counteract the effect of constant darkness (Hansson et al. 1993). Furthermore, a severe type of arthritis was developed in mice placed under constant light and immunized with type II collagen, whereas melatonin administration at disease onset (days 30–39) had no effect on the clinical signs of the disease (Hansson et al. 1992). In an adjuvant-induced arthritis model, a preventive and/or therapeutic therapy with the indoleamine lowers hind paw swelling in a comparable fashion to indomethacin (Chen and Wei 2002) in comparison to studies that showed the harmful effect of melatonin. Enhanced RA incidence and severity have been linked to higher latitudes, suggesting that increased melatonin production during long winter nights may be linked to RA. The development of RA is inversely related to UV-B exposure as these rays tend to decrease melatonin synthesis from pineal gland (Arkema et al. 2013). In individuals with RA, levels of nocturnal melatonin were higher in patients from Europe as compared to people from Italy (Cutolo et al. 2005). In early morning, the symptoms of RA get worsen (Cutolo and Masi 2005). Different studies have reported that individuals with RA had raised levels of sera melatonin in early morning in comparison to healthy individuals (Cutolo et al. 2005; Sulli et al. 2002). In individuals with RA, melatonin levels were significantly reduced in plasma (West and Oosthuizen 1992). In RA-cultured synovial macrophages, the sites for binding of melatonin molecule had higher affinity and intake of melatonin increased levels of NO and IL-12, respectively (Maestroni et al. 2002; Cutolo et al. 1999). The melatonin levels were higher synovial fluid of individuals with RA (Maestroni et al. 2002).
In young adults, Multiple sclerosis (MS) is the most prevalent neurological disorder with global incidence of 1.1–2.5 million cases and an increased worldwide incidence in middle-aged women. It is a neurodegenerative disease that is caused by an immunological response to myelin (Lassmann and Horssen 2011). Even though etiology of MS is still unknown, one of the environmental elements that appears to be involved is latitude, as the disease's incidence in northern countries increases (Kurtzke 1977). Because the incidence decreases in hilly areas compared to nearby lower areas (Kurtzke 1967), this has been linked to a reduction in sunshine exposure (van der Mei et al. 2003). Lately, shift work at an early age has been linked to an elevated risk of MS, with a positive association between MS risk and shift work length (Hedstrom et al. 2011). Melatonin and a MT6 circadian rhythms are both disrupted in MS patients. Melatonin had an inverted circadian rhythm in a large percentage of patients with worsened MS (Sandyk and Awerbuch 1992).
Systemic lupus erythematosus (SLE) is a complex autoimmune condition with an estimated incidence of 20–150 cases per 100,000 individuals and 1–10 new cases per 100,000 individuals yearly. In blood and tissues, the formation of immune complexes causes substantial tissular damage which is a prominent feature of SLE (Tsokos 2011). In the case of melatonin and lupus, a circadian rhythm uncoupling has been seen in lupus-prone mice (Lechner et al. 2000).
In mouse models of SLE, melatonin therapy has inconsistent effects, depending on the various criteria such as the administration timing and animal sex. Morning melatonin administration improved survival in lupus-prone mice, but the benefit was not replicated with evening treatment (Lenz et al. 1995). In kidney of female mice, melatonin supplementation effectively reduced vascular lesions and inflammatory infiltration, decreasing anti-collagen II and antidsDNA autoantibody titers, lowering production of pro-inflammatory cytokine, and increasing anti-inflammatory cytokine generation both in animals prone to lupus (Jimenez-Caliani et al. 2006) and in pristane-induced lupus (Zhou et al. 2010). Melatonin had little impact or aggravated the condition in male lupus-prone mice (Jimenez-Caliani et al. 2006).
Melatonin and Sepsis
Primarily sepsis is a microbial infection that causes systemic inflammation in the host body. Generally, microbial pathogens like bacteria, viruses, fungi, and other parasites (Annane et al. 2005; Calandra and Cohen 2005) cause it. It has been found that gram-negative bacteria possess lipopolysaccharide (LPS) in their cell wall that is ultimately been found to be responsible for sepsis initiation (Sriskandan and Cohen 1995). LPS causes significant induction of gene and expression of inflammatory molecules such as cytokines, chemokines, iNOS, and heat shock proteins by activating intracellular signaling pathways such as nuclear factor κB (Victor et al. 2004; Tsiotou et al. 2005). The natural balance between pro-inflammatory and anti-inflammatory mediators is broken during sepsis, resulting in the release of several inflammatory mediators (Pinsky 2001). In early phase of sepsis, soluble inflammatory molecules such as IL-1 and TNF-α are released. IL-1 causes ROS generation and protease stimulation, whereas cell activation is caused by TNF-α. In the onset of sepsis, mediators such as interferons and interleukins are involved. In both humans and animal models, melatonin supplementation has been effective in treating septic shock. The beneficial characteristics of melatonin in sepsis and bacterial infections are shown in Fig. 3.
Melatonin and Malaria
Malaria is one of the severe protozoal diseases that affects about 200 million people worldwide and kills over one million people each year (Sato 2021). Apart from Plasmodium falciparum, which in humans commonly causes malaria, a more known virulent parasite is Plasmodium knowlesi that rapidly spread not only in Malaysia but also throughout the world (White 2008; Yusof et al. 2014; Singh and Daneshvar 2013). In the past, a global malaria eradication initiative involving insecticide spraying yielded positive results. Malaria infections have been successfully eradicated thanks to potent antimalarial drugs, but with the increased drug resistance there is a reemergence of drug-resistant-associated malaria. Melatonin works as a trigger for Plasmodium falciparum growth and development, according to recent discoveries in malarial parasite genetic investigations (Mallaupoma et al. 2022). The same might be true for Plasmodium knowlesi. As a result, therapeutic strategies that successfully inhibit melatonin action on species of Plasmodium throughout the night involve therapy of bright light or blocking receptors of melatonin can be regarded as viable options for eradicating malaria in humans. The therapeutic implication of melatonin on Plasmodium falciparum cell cycle is represented in Fig. 4.
Melatonin in Vaccination
Vaccines are a significant effort to develop immunity to a specific disease and a growing attention toward enhancing the efficiency of preexisting vaccines. Vaccination’s immune-promoting efficacy is defined by antigenic part and appropriate adjuvants that are efficient in generating and supporting an effective immune response to pathogenic pathogens. Some in vivo research have looked into using melatonin as a vaccine agent, based on its immunoregulatory qualities. In sheep, lameness (Katz et al. 1991) is caused by Dichelobacter nodosus (Regodón et al. 2005) and the administration of melatonin enhanced humoral response in vaccinated sheep. The melatonin administered via injections or implants also increased response of platelets to thrombin stimulation thus improving aggregation rate, percentage, and lag time (Regodón et al. 2012). In sheep’s vaccinated with Clostridium perfringens type D, the administration of melatonin evoked beneficial immune response (Regodón et al. 2012). Interestingly, it was also found that immunization time also plays a vital part in imparting beneficial effects of melatonin as highest concentration of sera antibodies was found after vaccination prepartum. In vaccine development against prostrate cancer, the potential use of melatonin has been described; however, no data have been published till now (Connor 2008). Melatonin significantly improved the vaccine effectiveness of cercarial and soluble worm antigens, and also elevated GSH levels (Soliman et al. 2008). In the vaccination development against Alzheimer’s disease, melatonin use as a adjuvant has proved quite effective.
Melatonin and Reproduction
Melatonin is a potential molecule with wide variety of physiological properties and is known to affect reproductive status in different species (Thiéblot and Le Bars 1955). Before the discovery of melatonin, a study by Huebner in 1898 reported that the tumor of pineal gland in humans affected pubertal development and suggested that some molecule of pineal lineage influences reproductive function. Many researchers have investigated the relationship between the pineal and reproductive state in a number of species as a result of this finding, but few have been successful in proving a functional relationship (Thiéblot and Le Bars 1955). A study reported that in female rats that were administered with exogenous melatonin reduced ovarian weight (Wurtman et al. 1963). Ever since, there has been a plethora of evidence suggesting that reproductive function in numerous species is influenced by the pineal gland, via melatonin (Reiter 1993). There is substantial evidence that the photoperiod-mediated frequency of melatonin secretion has a direct impact on reproductive capacity. Melatonin’s key physiological purpose is to describe the daily light/dark (LD) cycle. The potential impact of melatonin on reproductive functions is shown in Fig. 5.
Melatonin in Mood Disorders
The role of melatonin has been found in the development of various mood disorders such as bipolar disorder (BD), major depressive disorder (MDD), and seasonal affective disorder (SAD). The major abnormalities associated with BD patients are the disturbances in sleep especially reduced sleep during night and abnormal circadian rhythms (Harvey 2008; Mansour et al. 2005). In individuals affected with BD, a marked decrease in secretion of melatonin is seen in depressed stage, and with symptoms remission, it was restored to normal. In individuals with MDD disturbances like wakefulness in early morning hours, mood, alertness, and fatigue during day time are more commonly known. The individuals with MDD have increased levels of melatonin in their body, and a phase wise shift of this hormone is considered as the utmost clinical feature of this disorder. In SAD, patients during winter season experience depression episodes and euthymia in summer time. The seasonal variations of melatonin are found in SAD individuals. In the pathogenesis of mood disorders, most of the patients suffer from disturbances in circadian rhythms and sleep. In individuals with several mood disorders, altered melatonin levels cause abnormalities in the biological clock functioning located in suprachiasmatic nuclei of anterior hypothalamus. Hence, treatment procedures should be developed on using antidepressant medications that can restore circadian clock functioning, and in this context, Agomelatine has been found to be quite effective as an antidepressant drug. Agomelatine is a chemically synthesized naphthalenic compound that primarily acts on MT1 and MT2 receptors. The half-life of this drug is higher than melatonin and is primarily metabolized in the liver via CYP isoenzymes (CYPA1, CYPA2, and CYP2C9). Agomelatine shows no significant affinity against receptors of adrenergic, dopaminergic, muscarinic, and histaminergic. In the treatment of mood disorders, Agomelatine is quite useful as it resynchronizes abnormal circadian rhythms and disturbed patterns of sleep (Kennedy et al. 2011). In addition to antidepressant property of Agomelatine, no adverse effects like disturbances in sleep patterns, discontinuation of medications, and sexual dysfunction were not found. Agomelatine has also found to be quite efficient in treating obsessive compulsory disorders (Fornaro 2011). In recent times, a number of research studies have reported that Agomelatine shows good clinical efficacy, safety, and tolerability (Rouillon 2006; Kennedy and Rizvi 2010).
Melatonin in Transplantation
The role of melatonin has also been found in prolonged survival of graft as beneficial in animal models. In a rat model with cardiac transplant, melatonin (200 mg/kg) was found to inhibit allograft immune response as it reduced proliferation of lymphocytes thus stopping rejection and increasing survival of graft (Jung et al. 2004). Simultaneously, after prolonged ischemia in lungs with reperfusion injury similar effects were observed (Inci et al. 2002). In non-obese diabetic mice, high administration of melatonin predominantly enhanced survival of islet graft as it inhibited T cell proliferation and increased IL-10 cell population (Lin et al. 2009). In a rat model with ovary transplantation, the melatonin administration depicted immunosuppressive property (Sapmaz et al. 2003). A comparable research study of ovarian grafts in humans treated with melatonin showed reduced apoptosis (Friedman et al. 2012). In ischemia–reperfusion injured animal models, protective effects of melatonin administration have been reported (Fildes et al. 2009). The intake of melatonin caused reduction in apoptotic cells, oxidative molecules, recruitment of neutrophils, and also raised GSH (Baykara et al. 2009).
Melatonin and Other Infections
During past century, melatonin has been considered as a significant antibiotic, anti-parasitic, and antiviral entity (Bagnaresi et al. 2012). The protective role of melatonin has been found against Venezuelan equine encephalomyelitis virus (VEEV) that causes viral infections (Montiel et al. 2015). From 1920 to 1970, the outbreaks of this viral infection occurred in northern part of South America wherein thousands of people, donkeys, and horses were affected (Bowen and Calisher 1976). This infection also resurfaced in 1995 and caused mortality in the affected area (Weaver et al. 1996). This infection caused excitation and hypermotility, subsequently followed by hypomotility, coma, paralysis, and death. In mice, a series of experimental studies with the infected virus were carried out and after melatonin administration the disease onset was delayed with reduced mortality rate as the viral load decreased in brain and blood (Bonilla et al. 1997). In immunocompetent mice, VEEV levels were reduced in brain by melatonin treatment as compared to immunodepressed mice, proposing that for depicting antiviral activity melatonin requires immune system integrity (Bonilla et al. 2001). In encephalitis, a disease caused by pathogenic encephalomyocarditis virus (EMCV), the melatonin supplementation in mice prevented paralysis and death in rodents infected with EMCV sublethal doses (Wongchitrat et al. 2010). A study reported that Semliki forest virus (SFV)-infected mice had defects in central nervous system and causes death, the administration of melatonin decreased viremia, delayed disease onset, and reduced mortality (Carrillo-Vico et al. 2013). In various developed models of bacterial infections, melatonin seems to have a potent role in scavenging of toxic free radicals and also antioxidant properties (Manchester et al. 2015). In M. tuberculosis, few studies have reported seasonality in infection cases which peaks at start of summer and end of winter season (Liao et al. 2019). All of the seasonality changes have been reported, and few researchers have proposed that annual variations in the melatonin concentrations cause seasonal variations in the immune system (Ozkank et al. 2012). The melatonin supplementation has found to be quite efficient in treating parasitic infections.
Melatonin and Pain
The potential damage to a tissue causes pain that is an unpleasant sensory feeling. The perception of pain mechanism is a multifactorial event consisting of biochemical, neurophysiological, humoral, and psychological parameters (Shavali et al. 2005). During tissue damage, a range of inflammatory molecules such as cytokines, prostaglandins, TNF-α, bradykinin, and leukotrienes are released into the blood circulation. These inflammatory substances act either directly or release agents that act on receptors that promote excitability of neurons involved in pathways of pain transmission (Meyer 2006). To control and manage, pain is a subject of great concern and interest and various drugs are possibly used for this purpose. Most of the drugs used whether non-steroidal anti-inflammatory drugs or aspirin show their effect by inhibiting cyclooxygenases (Fokunang et al. 2018). In humans and rodents, pain perceptions with diurnal variations have been described (Tappe‐Theodor and Kuner 2014). It has been found that during dark phase, individuals experienced less pain, whereas in healthy human cases, prolonged delay in pain levels was reported (Tappe‐Theodor and Kuner 2014). This phenomenon was possibly attributed to elevated levels of melatonin at night, and with this observation, a number of experimental designs and models were developed. The antinociceptive property of melatonin as reported by early studies demonstrates the involvement of benzodiazepinergic (BZD) and opiate pathways. Besides these, melatonin influences its effects via neurotransmitter systems and associated receptor sites sigma system, BZD, adrenergic, serotonergic, glutamatergic, dopaminergic, and directly via MT1/MT2 receptors (Mantovani et al. 2006). Antinociceptive property is also exerted by nitric oxide molecule interacting via cyclooxygenase (COX) and NMDA receptors (Cury et al. 2011).
Melatonin and Polycystic Ovary Syndrome (PCOS)
Polycystic ovary syndrome (PCOS) is a complex endocrine disorder affecting 20% of women at reproductive age (Shaikh et al. 2014). PCOS occurs due to the interaction of various environmental and genetic factors. The clinical features associated with PCOS are hyperandrogenism, anovulatory infertility, menstrual irregularity, and obesity and due to these complaints women often seek treatment. Despite the recent developments made in the understanding and treatment procedures over the years, many questions still remain unanswered; however, changing lifestyle including weight loss and nutritional counseling are included in treatment plan. The oocytes, follicular cells, and cytotrophoblasts are some of the sites of melatonin production in the female reproductive system. The receptors of melatonin in the ovary and intra-follicular fluid maintain the secretion of sex steroid at various phases of follicular maturation. During the follicular maturation, melatonin protects the ovarian follicles as it is a strong antioxidant and scavenges free radicals effectively. In the female reproductive physiology, the melatonin effects are mediated via the receptors located in ovarian, hypothalamic, and pituitary sites (Reiter et al. 2009). Melatonin has been known to protect oocytes and developing fetus from oxidative stress (Tan et al. 2003). In women affected with PCOS, the levels of melatonin have been measured by various studies to know about its role in pathogenesis (Luboshitzky et al. 2003, 2004). Some research studies have suggested that the administration of exogenous melatonin improves glucose hemostasis and endothelial vascular function in experimental animal models (Pai and Majumdar 2014). In PCOS individuals, the melatonin intake imparts protective features against reproductive and metabolic abnormalities.
Melatonin Agonists
As melatonin hormone possesses various beneficial properties as a result of which many agonists of it have been synthesized. In numerous pathologies, a combination of melatonin with other drugs has proven to be quite useful. The therapeutic potential of melatonin molecule is limited because of its short half-life in circulation. Some of the agonists of melatonin are depicted below.
Agomelatine
It is synthetic and effectively potential agonist of MT1/MT2 receptors. A > 100 fold selectivity is shown by Agomelatine to MT1/MT2 receptors. The half-life of Agomelatine is higher (2 h) as compared to melatonin. In more than 40 countries of the world, this drug is available now in the market and is mostly used for treatment of adult depression (Fornaro 2011; Carney and Shelton 2011). In patients with depressive disorders, this drug relives symptoms and restores circadian rhythms. Even though the clinical aim of this drug was to target moderate to severe depression, however, this agonist of melatonin has been found to be quite effective in treating GAD, SAD, and BD (da Rocha and Correa 2011). The chronobiotic and sleep inducing effects of Agomelatine are due to its melatonergic receptors located in SCN. In all the research studies conducted so far, Agomelatine has shown good safety and tolerability.
Tasimelteon
A non-selective agonist of MT1/MT2 receptor family is Tasimelteon drug. This drug has successfully passed Phase III trials where it was found quite effective in improving maintenance and onset of sleep with minimal side effects (Arendt and Rajaratnam 2008).
Ramelteon
In 2005, FDA approved this drug for treating sleep disorders, insomnia, and other alterations in sleep (Cajochen 2005). This drug is quite selective agonist with high affinity (3–16 times than melatonin) for receptors of MT1/MT2 (Kato et al. 2005). In patients of insomnia with different age groups (18–83 years), this drug was found to elevate total sleep duration in affected individuals (Greenblatt et al. 2007). Furthermore, this drug showed no affinity to MT3, dopamine, benzodiazepine, opiate, and serotonin receptors (Zammit et al. 2009). In the circulation, the half-life of Ramelteon is much longer (1–2 h) than melatonin. In the gastrointestinal tract, about 84% of Ramelteon is quickly absorbed.
Piromelatine
This drug is agonist of 5HT1A, 5HT1D, and 5HT2B and is still under development with studies in rodent animal models. This drug has completed Phase II study and its target includes treatment of insomnia, anxiolytic, and antidepressant effects.
Conclusion
Melatonin is a ubiquitous molecule with wide distribution in nature and is synthesized by animals, humans, plants, fungi, and unicellular organisms. Biologically melatonin mostly acts through G-protein-coupled receptors located in the plasma membrane with MT1 and MT2 being the two functional receptors. All mammals express these in different organs of the body including the humans. A wide range of functions is displayed by melatonin like immune regulating, antioxidant, reproduction, puberty timing, hypnotic, oncostatic, mood, behavior, pain and chronobiotic. Deficiencies in the production of melatonin or impaired receptor expression have been linked to various anomalies like obesity, breast cancer, neurological disorders, diabetes, hypertension, prostate cancer, autoimmune disorders, mood disorders, and transplantation. The involvement of melatonin molecule in chronic insomnia and sleep disorders has been found. In decreasing oxidative damage, melatonin function seems to be quite vital. To prevent and treat various disorders, the administration of exogenous melatonin has been used by many clinical trials. Many melatonin agonists have been synthesized and developed with the aim to treat various disorders. A novel clinical area has been formed with the development of new melatonin agonists as they possess better pharmacokinetics. Thus, melatonin is a quite important hormonal molecule in the biological living world.
Data Availability
All the data generated have been presented in this article.
Abbreviations
- ASMT:
-
Acetyl-serotonin-methyltransferase
- NAT:
-
N-Acetyltransferase
- RZR/ROR:
-
Retinoid-related Orphan nuclear hormone receptor family
- GPCR:
-
G-protein coupled receptor
- GnRH:
-
Gonadotrophin-releasing hormone
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- AD:
-
Alzheimer’s disease
- Aβ:
-
β-Amyloid
- APP:
-
Amyloid precursor protein
- PD:
-
Parkinson’s disease
- SNC:
-
Substantia nigra pars compacta
- T2D:
-
Type 2 diabetes
- ZDF:
-
Zucker diabetic fatty
- Glut 1 and Glut 2:
-
Glucose transporters
- RA:
-
Rheumatoid arthritis
- MS:
-
Multiple sclerosis
- SLE:
-
Systemic lupus erythematosus
- BD:
-
Bipolar disorder
- MDD:
-
Major depressive disorder
- SAD:
-
Seasonal affective disorder
- BZD:
-
Benzodiazepinergic
- TNF-α:
-
Tumor necrosis factor-α
- IL-1β:
-
Intereukin-1β
- MT1:
-
Melatonin receptor 1
- MT2:
-
Melatonin receptor 2
References
Agil A, Rosado I, Ruiz R, Figueroa A, Zen N, Fernandez-Vazquez G (2012) Melatonin improves glucose homeostasis in young Zucker diabetic fatty rats. J Pineal Res 52:203–210
Ali A, Dar MA, Ayaz A (2017a) Diagnostic approaches to diabetes mellitus and the role of vitamins. J Nutr Food Sci. https://doi.org/10.4172/2155-9600.1000601
Ali A, Ayaz A, Dar MA, Singh N, Bhat SA, Razak R (2017b) A key role of insulin in diabetes mellitus. Int J Sci Res Sci 3:80–85
Amaral FGD, Cipolla-Neto J (2018) A brief review about melatonin, a pineal hormone. Arch Endocrinol Metab 62:472–479
Anisomov VN, Arutjunyan V, Khavinson VK (2001) Effects of pineal peptide preparation epithalamin on free radical processes in humans and animals. Neuroendocrinol Lett 22:9–18
Annane D, Bellissant E, Cavallion JM (2005) Septic shock. Lancet 365:63–78
Antolin I, Mayo JC, Sainz RM, de los Angeles del Brio L, Herrera F, Martin V, Rodriguez C (2002) Protective effect of melatonin in a chronic experimental model of Parkinson’s disease. Brain Res 943:163–173
Anwar MM, Meki AR, Abu Rahman HH (2001) Inhibitory effects of melatonin on vascular reactivity, possible role of vasoactive mediators. Comp Biochem Physiol 130:357–367
Arendt J (2006) Melatonin and human rhythms. Chronobiol Int 23:21–37
Arendt J, Rajaratnam SM (2008) Melatonin and its agonists, an update. Br J Psychiatry 193:267–269
Arkema EV, Hart JE, Bertrand KA, Laden F, Grodstein F, Rosner BA (2013) Exposure to ultraviolet-B and risk of developing rheumatoid arthritis among women in the Nurses’ Health Study. Ann Rheum Dis 72:506–511
Back K, Tan DX, Reiter RJ (2016) Melatonin biosynthesis in plants, multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J Pineal Res 61:426–437
Bagnaresi P, Nakabashi M, Thomas AP, Reiter RJ, Garcia CR (2012) The role of melatonin in parasite biology. Mol Biochem Parasitol 181:1–6
Barlow-Walden LR, Reiter RJ, Abe M, Pablos M, Menendez-Pelaez A, Chen LD, Poeggeler B (1995) Melatonin stimulates brain glutathione peroxidase activity. Neurochem Int 26:497–502
Barrenetxe J, Delagrange P, Martinez JA (2004) Physiological and metabolic functions of melatonin. J Physiol Biochem 60:61–72
Bartness TJ, Wade GN (1985) Body weight, food intake and energy regulation in exercising and melatonin-treated siberian hamsters. Physiol Behav 35:805–808
Baykara B, Tekmen I, Pekcetin C, Ulukus C, Tuncel P, Sagol O (2009) The protective effects of carnosine and melatonin in ischemia-reperfusion injury in the rat liver. Acta Histochem 111:42–51
Bazwinsky-Wutschke I, Bieseke L, Muhlbauer E, Peschke E (2014) Influence of melatonin receptor signalling on parameters involved in blood glucose regulation. J Pineal Res 56:82–96
Benitez-King G, Rıos A, Martınez A, Anton-Tay F (1996) In vitro inhibition of Ca2+/calmodulin-dependent kinase II activity by melatonin. Biochim Biophys Acta 1290:191–196
Bhat MA, Bhat SA, Ahmad SB, Qureshi W, Majid S, Ali A, Hussain I, Hassan T, Rehman MU, Mir MR (2017) Biochemical profile and genetic polymorphism of MTHFRC677T in risk of type 2 diabetes mellituss. Int J Diabetes Endocrinol 2:19–25
Binkley S, Mosher K, Rubin F, White B (1988) Xenopus tadpole melanophores are controlled by dark and light and melatonin without influence of time of day. J Pineal Res 5:87–97
Bolliet V, Ali MA, Lapointe FJ, Falcon J (1996) Rhythmic melatonin secretion in different teleost species, an in vitro study. J Comp Physiol B 165:677–683
Bonilla E, Valero-Fuenmayor N, Pons H, ChacinBonilla L (1997) Melatonin protects mice infected with Venezuelan equine encephalomyelitis virus. Cell Mol Life Sci 53:430–434
Bonilla E, Rodon C, Valero N, Pons H, Chacin-Bonilla L, Tamayo JG (2001) Melatonin prolongs survival of immunodepressed mice infected with the Venezuelan equine encephalomyelitis virus. Trans R Soc Trop Med Hyg 95:207–10
Bowen GS, Calisher CH (1976) Virological and serological studies of Venezuelan equine encephalomyelitis in humans. J Clin Microbiol 4:22–27
Bray MS, Young ME (2012) Chronobiological effects on obesity. Curr Obes Rep 1:9–15
Brugger P, Marktl W, Herold M (1995) Impaired secretion of melatonin in coronary heart disease. Lancet 345:1408
Brzezinski A (1997) Melatonin in humans. N Eng J Med 336:186–195
Bubenik GA (2002) Gastrointestinal melatonin, localization, function, and clinical relevance. Dig Dis Sci 47:2336–2348
Buijs RM, La Fleur SE, Wortel J (2003) The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J Comp Neurol 464:36–48
Burkhardt S, Tan DX, Manchester LC, Hardeland R, Reiter RJ (2001) Detection and quantification of the antioxidant melatonin in Montmorency and Balaton tart cherries (Prunus cerasus). J Agric Food Chem 49:4898–4902
Buswell RS (1975) The pineal and neoplasia. Lancet 1:34–35
Byeon Y, Lee HY, Hwang OJ, Lee HJ, Lee K, Back K (2015) Coordinated regulation of melatonin synthesis and degradation genes in rice leaves in response to cadmium treatment. J Pineal Res 58:470–478
Cabrera J, Negrín G, Estévez F, Loro J, Reiter RJ, Quintana J (2010) Melatonin decreases cell proliferation and induces melanogenesis in human melanoma SK-MEL-1 cells. J Pineal Res 49:45–54
Cajochen C (2005) TAK-375 Takeda. Curr Opin Investig Drugs 6:114–121
Calandra T, Cohen J (2005) The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit Care Med 33:1538–1548
Capsoni S, Viswanathan M, De Oliveira AM, Saavedra JM (1994) Characterization of melatonin receptors and signal transduction system in rat arteries forming the Circle of Willis. Endocrinology 135:373–378
Cardinali DP, Rosner JM (1971) Metabolism of serotonin by the rat retina in vitro. J Neurochem 18:1769–1770
Cardinali DP, Golombek DA, Rosenstein RE, Cutrera RA, Esquifino AI (1997) Melatonin site and mechanism of action, single or multiple? J Pineal Res 23:32–39
Cardinali DP, Pagano ES, Scacchi Bernasconi PA, Reynoso R, Scacchi P (2011) Disrupted chronobiology of sleep and cytoprotection in obesity, possible therapeutic value of melatonin. Neuroendocrinol Lett 32:588–606
Carlberg C (2000) Gene regulation by melatonin. Ann N Y Acad Sci 917:387–396
Carney RM, Shelton RC (2011) Agomelatine for the treatment of major depressive disorder. Expert Opin Pharmacother 12:2411–2419
Carrillo-Vico A, Garcia-Perganeda A, Naji L (2003) Expression of membrane and nuclear melatonin receptor mRNA and protein in the mouse immune system. Cell Mol Life Sci 60:2272–2278
Carrillo-Vico A, Lardone PJ, Álvarez-Sánchez N, Rodríguez-Rodríguez A, Guerrero JM (2013) Melatonin: buffering the immune system. Int J Mol Sci 2013(14):8638–8683
Chen Q, Wei W (2002) Effects and mechanisms of melatonin on inflammatory and immune responses of adjuvant arthritis rat. Int Immunopharmacol 2:1443–1449
Chen G, Huo Y, Tan DX, Liang Z, Zhang W, Zhang Y (2003) Melatonin in Chinese medicinal herbs. Life Sci 73:19–26
Chen TY, Lee MY, Chen HY, Kuo YL, Lin SC, Wu TS, Lee EJ (2006) Melatonin attenuates the postischemic increase in blood–brain barrier permeability and decreases hemorrhagic transformation of tissue-plasminogen activator therapy following ischemic stroke in mice. J Pineal Res 40:242–250
Chu LW, John EM, Yang B, Kurian AW, Zia Y, Yu K (2018) Measuring serum melatonin in postmenopausal women, implications for epidemiologic studies and breast cancer studies. PLoS ONE 13:e0195666
Cipolla-Neto J, Amaral FGD (2018) Melatonin as a hormone: new physiological and clinical insights. Endocr Rev 39:990–1028
Clemens JW, Jarzynka MJ, Witt-Enderby PA (2001) Down-regulation of MT1 melatonin receptors in rat ovary following estrogen exposure. Life Sci 69:27–35
Comai S, Gobbi G (2014) Unveiling the role of melatonin MT2 receptors in sleep, anxiety and other neuropsychiatric diseases, a novel target in psychopharmacology. J Psychiatry Neurosci 39:6–21
Connor TP (2008) Melatonin as an adjuvant to therapeutic prostate cancer vaccines. J Pineal Res 45:224
Cook JS, Sauder CL, Ray CA (2011) Melatonin differentially affects vascular blood flow in humans. Am J Physiol Heart Circ Physiol 300:H670–H674
Coon SL, Weller JL, Korf HW (2001) cAMP regulation of arylalkylamine N-acetyltransferase (AANAT, EC 2.3.1.87), a new cell line (1e7) provides evidence of intracellular AANAT activation. J Biol Chem 276:24097–107
Cos S, Sanchez-Barcelo EJ (2000) Melatonin and mammary pathological growth. Front Neuroendocrinol 21:133–170
Cury Y, Picolo G, Guiterrez VP, Ferreira SH (2011) Pain and analgesia, the dual effect of nitric oxide in the nociceptive system. Nitric Oxide 25:243–254
Cutolo M, Masi AT (2005) Circadian rhythms and arthritis. Rheum Dis Clin N Am 31:115–129
Cutolo M, Villaggio B, Candido F, Valenti S, Giusti M, Felli L (1999) Melatonin influences interleukin-12 and nitric oxide production by primary cultures of rheumatoid synovial macrophages and THP-1 cells. Ann N Y Acad Sci 876:246–254
Cutolo M, Maestroni GJ, Otsa K, Aakre O, Villaggio B, Capellino S (2005) Circadian melatonin and cortisol levels in rheumatoid arthritis patients in winter time, a north and south Europe comparison. Ann Rheum Dis 64:212–216
da Rocha FF, Correa H (2011) Is circadian rhythm disruption important in obsessive–compulsive disorder (OCD)? A case of successful augmentation with agomelatine for the treatment of OCD. Clin Neuropharmacol 34:139–140
Daniels WM, van Rensburg SJ, van Zyl JM, Taljaard JJ (1998) Melatonin prevents beta-amyloid-induced lipid peroxidation. J Pineal Res 24:78–82
de la Puerta C, Carrascosa-Salmoral MP, García-Luna PP, Lardone PJ, Herrera JL, Fernández-Montesinos R (2007) Melatonin is a phytochemical in olive oil. Food Chem 104:60912
Dehghan F, Khaksari Hadad M, Asadikram G, Najafipour H, Shahrokhi N (2013) Effect of melatonin on intracranial pressure and brain edema following traumatic brain injury, role of oxidative stresses. Arch Med Res 44:251–258
Donga E, van Dijk M, van Dijk JG, Biermasz NR, Lammers GJ, van Kralingen KW (2010) A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. J Clin Endocrinol Metab 95:2963–2968
Doolen S, Krause DN, Dubocovich ML, Duckles SP (1998) Melatonin mediates two distinct responses in vascular smooth muscle. Eur J Pharmacol 345:67–69
Dubocovich ML (1995) Melatonin receptors, are there multiple subtypes? Trends Pharmacol Sci 16:50–56
Dubocovich ML, Yun K, Al-Ghoul WM, Benloucif S, Benloucif S, Masana MI (1998) Selective MT2 melatonin receptor antagonists block melatoninmediated phase advances of circadian rhythms. FASEB J 12:1211–1220
Dubocovich ML, Rivera-Bermudez MA, Gerdin MJ, Masana MI (2003) Molecular pharmacology, regulation and function of mammalian melatonin receptors. Front Biosci 8:d1093-1108
Ebisawa T, Karne S, Lerner MR, Reppert SM (1994) Expression cloning of a high-affinity melatonin receptor from Xenopus dermal melanophores. Proc Natl Acad Sci USA 91:6133–6137
Ekmekcioglu C (2006) Melatonin receptors in humans, biological role and clinical relevance. Biomed Pharmacother 60:97–108
Elbaz A, Moisan F (2008) Update in the epidemiology of Parkinson’s disease. Curr Opin Neurol 21:454–460
Ersahin M, Toklu HZ, Cetinel S, Yuksel M, Yegen BC, Sener G (2009) Melatonin reduces experimental subarachnoid hemorrhageinduced oxidative brain damage and neurological symptoms. J Pineal Res 46:324–332
Fan C, Pan Y, Yang Y, Di S, Jiang S, Ma Z (2015) HDAC1 inhibition by melatonin leads to suppression of lung adenocarcinoma cells via induction of oxidative stress and activation of apoptotic pathways. J Pineal Res 59:321–333
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H (2012) Cancer incidence and mortality patterns in Europe, estimates for 40 countries in 2012. Eur J Cancer 49:1374–1403
Fildes JE, Yonan N, Keevil BG (2009) Melatonin—a pleiotropic molecule involved in pathophysiological processes following organ transplantation. Immunology 127:443–449
Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre MF (2015) The global burden of cancer 2013. JAMA Oncol 1:505–527
Flynn RW, MacWalter RS, Doney AS (2008) The cost of cerebral ischaemia. Neuropharmacology 55:250–256
Fokunang C, Fokunang ET, Frederick K, Ngameni B, Ngadjui B (2018) Overview of non-steroidal anti-inflammatory drugs (NSAIDs) in resource limited countries. MOJ Toxicol 4:5–13
Fonken LK, Nelson RJ (2014) The effects of light at night on circadian clocks and metabolism. Endocr Rev 35:648–670
Foon KA (1989) Biological response modifiers, the new immunotherapy. Cancer Res 49:1621–1627
Forman JP, Curhan GC, Schernhammer ES (2010) Urinary melatonin and risk of incident hypertension among young women. J Hypertens 28:446–451
Fornaro M (2011) Switching from serotonin reuptake inhibitors to agomelatine in patients with refractory obsessive–compulsive disorder, a 3 month follow-up case series. Ann Gen Psychiatry 10:5
Friedman O, Orvieto R, Fisch B, Felz C, Freud E, Ben-Haroush A (2012) Possible improvements in human ovarian grafting by various host and graft treatments. Hum Reprod 27:474–82
Galano A, Tan DX, Reiter RJ (2013) On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J Pineal Res 54:245–257
Galano A, Medina ME, Tan DX, Reiter RJ (2015) Melatonin and its metabolites as copper chelating agents and their role in inhibiting oxidative stress, a physicochemical analysis. J Pineal Res 58:107–116
Garcia JJ, Lopez-Pingarron L, Almeida-Souza P, Tres A, Escudero P, Garcıa-Gil FA, Tan DX, Reiter RJ, Ramırez JM, Bernal-Perez M (2014) Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes, a review. J Pineal Res 56:225–237
Gatti G, Lucini V, Dugnani S, Calastretti A, Spadoni G, Bedini A (2017) Antiproliferative and pro-apoptotic activity of melatonin analogues on melanoma and breast cancer cells. Oncotarget 8:68338–68353
Gilgun-Sherki Y, Rosenbaum Z, Melamed E, Offen D (2002) Antioxidant therapy in acute central nervous system injury, current state. Pharmacol Rev 54:271–284
Gilman SC, Bonner MJ, Pellmar TC (1993) Effect of oxidative stress on excitatory amino acid release by cerebral cortical synaptosomes. Free Radic Biol Med 15:671–675
Girouard H, Denault C, Chulak CH (2004) Treatment by N-acetylcysteine and melatonin increase cardiac baroreflex and improves antioxidant reserve. Am J Hypertens 17:947–954
Greenblatt DJ, Harmatz JS, Karim A (2007) Age and gender effects on the pharmacokinetics and pharmacodynamics of ramelteon, a hypnotic agent acting via melatonin receptors MT1 and MT2. J Clin Pharmacol 47:485–496
Grima NA, Rajaratnam SM, Mansfield D, Sletten TL, Spitz G, Ponsford JL (2018) Efficacy of melatonin for sleep disturbance following traumatic brain injury: a randomised controlled trial. BMC Med 2018(16):1–10
Grossman E, Laudon M, Yalcin R (2006) Melatonin reduces night blood pressure in patients with nocturnal hypertension. Am J Med 119:898–902
Grossman E, Laudon M, Zisapel N (2011) Effect of melatonin on nocturnal blood pressure, meta-analysis of randomized controlled trials. Vasc Health Risk Manag 7:577–584
Gunata M, Parlakpinar H, Acet HA (2020) Melatonin: a review of its potential functions and effects on neurological diseases. Rev Neurol 176:148–165
Gupta YK, Gupta M, Kohli K (2003) Neuroprotective role of melatonin in oxidative stress vulnerable brain. Indian J Physiol Pharmacol 47:373–386
Hajam YA, Rai S (2019) Melatonin and insulin modulates the cellular biochemistry, histoarchitecture and receptor expression during hepatic injury in diabetic rats. Life Sci 239:117046
Hajam YA, Rai S, Pandi-Perumal SR, Brown GM, Reiter RJ, Cardinali DP (2022) Coadministration of melatonin and insulin improves diabetes-induced impairment of rat kidney function. Neuroendocrinology 112:807–822
Hansson I, Holmdahl R, Mattsson R (1992) The pineal hormone melatonin exaggerates development of collagen-induced arthritis in mice. J Neuroimmunol 39:23–30
Hansson I, Holmdahl R, Mattsson R (1993) Pinealectomy ameliorates collagen II-induced arthritis in mice. Clin Exp Immunol 92:432–436
Hardeland R (2012) Melatonin in aging and disease-multiple consequences of reduced secretion, options and limits of treatment. Aging Dis 3:194–225
Harvey AG (2008) Sleep and circadian rhythms in bipolar disorder, seeking synchrony, harmony, and regulation. Am J Psychiatry 165:820–829
Hattori A, Migitaka H, Iigo M, Itoh M, Yamamoto K, OhtaniKaneko R (1995) Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem Mol Biol Int 35:62734
He H, Dong W, Huang F (2010) Anti-amyloidogenic and anti-apoptotic role of melatonin in Alzheimer disease. Curr Neuropharmacol 8:211–217
Hedstrom AK, Akerstedt T, Hillert J, Olsson T, Alfredsson L (2011) Shift work at young age is associated with increased risk for multiple sclerosis. Ann Neurol 70:733–741
Hissa MN, Lima GG, Simões JC, Nunes RTL (2008) Melatonin and pineal gland. Rev Elect Pesq Méd 2:1–10
Hughes KC, Gao X, Kim IY, Wang M, Weisskopf MG, Schwarzschild MA, Ascherio A (2017) Intake of dairy foods and risk of Parkinson disease. Neurology 89:46–52
Hussain SA (2007) Effect of melatonin on cholesterol absorption in rats. J Pineal Res 42:267–271
Inci I, Inci D, Dutly A, Boehler A, Weder W (2002) Melatonin attenuates posttransplant lung ischemia–reperfusion injury. Ann Thorac Surg 73:220–225
Iriti M, Varoni EM, Vitalini S (2010) Melatonin in traditional Mediterranean diets. J Pineal Res 49:1015
Ittner LM, Gotz J (2011) Amyloid-beta and tau—a toxic pas de deux in Alzheimer’s disease. Nat Rev Neurosci 12:65–72
James LJ, Wong G, Craig JC, Hanson CS, Ju A, Howard K (2017) Men’s perspectives of prostate cancer screening, a systematic review of qualitative studies. PLoS ONE 12:e0188258
Jang SW, Liu X, Pradoldej S (2010) N-Acetylserotonin activates Trkb receptor in a circadian rhythm. Proc Natl Acad Sci USA 107:3876–3881
Jeong S (2017) Molecular and cellular basis of neurodegeneration in Alzheimer’s disease. Mol Cells 40:613–620
Jimenez-Caliani AJ, Jimenez-Jorge S, Molinero P, Fernandez-Santos JM, Martin-Lacave I, Rubio A (2006) Sex-dependent effect of melatonin on systemic erythematosus lupus developed in Mrl/Mpj-Faslpr mice, it ameliorates the disease course in females, whereas it exacerbates it in males. Endocrinology 147:1717–1724
Jockers R, Maurice P, Boutin JA, Delagrange P (2008) Melatonin receptors, heterodimerization, signal transduction and binding sites: what’s new? Br J Pharmacol 154(6):1182–1195
Jockers R, Delagrange P, Dubocovich ML, Markus RP, Renault N, Tosini G (2016) Update on melatonin receptors, IUPHAR review 20. Br J Pharmacol 173:2702–2725
Jou MJ, Jou SB, Chen HM, Lin CH, Peng TI (2002) Critical role of mitochondrial reactive oxygen species formation in visible laser irradiation-induced apoptosis in rat brain astrocytes (RBA-1). J Biomed Sci 9:507–516
Jung FJ, Yang L, Harter L, Inci I, Schneiter D, Lardinois D (2004) Melatonin in vivo prolongs cardiac allograft survival in rats. J Pineal Res 37:36–41
Kanishi Y, Kobayashi Y, Noda S, Ishizuka B, Saito K (2000) Differential growth inhibitory effect of melatonin on two endometrial cancer cell lines. J Pineal Res 28:227–233
Karolczak K, Watala C (2021) Melatonin as a reducer of neuro- and vasculotoxic oxidative stress induced by homocysteine. Antioxidants 10:1178
Kato K, Hirai K, Nishiyama K, Uchikawa O, Fukatsu K, Ohkawa S (2005) Neurochemical properties of ramelteon (TAK-375), a selective MT1/MT2 receptor agonist. Neuropharmacology 48:301–310
Katz ME, Howarth PM, Yong WK, Riffkin GG, Depiazzi LJ, Rood JI (1991) Identification of three gene regions associated with virulence in Dichelobacter nodosus, the causative agent of ovine footrot. J Gen Microbiol 137:2117–2124
Kennedy SH, Rizvi SJ (2010) Agomelatine in the treatment of major depressive disorder, potential for clinical effectiveness. CNS Drugs 24:479–499
Kennedy SH, Young AH, Blier P (2011) Strategies to achieve clinical effectiveness, refining existent therapies and pursuing emerging targets. J Affect Disord 132:S21–S28
Klein DC (1985) Photoneural regulation of the mammalian pineal gland. In: Evered D, Clark S (eds) Photoperiodism, melatonin and the pineal. Ciba Foundation symposium, vol 117. Pitman, London, pp 38–56
Kosar PA, Naziroglu M, Ovey ÝS, Çig B (2016) Synergic effects of doxorubicin and melatonin on apoptosis and mitochondrial oxidative stress in MCF-7 breast cancer cells, involvement of TRPV1 channels. J Membr Biol 249:129–140
Krause DN, Barrios VE, Duckles SP (1995) Melatonin receptors mediate potentiation of contractile responses to adrenergic nerve stimulation in rat caudal artery. Eur J Pharmacol 276:207–213
Kurcer Z, Sahna E, Olmez E (2006) Vascular reactivity to various vasoconstrictor agents and endotheliumdependent relaxations of rat thoracic aorta in the long-term period of pinealectomy. J Pharmacol Sci 101:329–334
Kurtzke JF (1967) On the fine structure of the distribution of multiple sclerosis. Acta Neurol Scand 43:257–282
Kurtzke JF (1977) Geography in multiple sclerosis. J Neurol 215:1–26
Lahiri DK (1999) Melatonin affects the metabolism of the betaamyloid precursor protein in different cell types. J Pineal Res 26:137–146
Lai SW, Liu YS, Lu DY, Tsai CF (2019) Melatonin modulates the microenvironment of glioblastoma multiforme by targeting sirtuin 1. Nutrients 11:1343
Larkin LM, Moore BJ, Stern JS, Horwitz BA (1991) Effect of photoperiod on body weight and food intake of obese and lean Zucker rats. Life Sci 49:735–745
Lassmann H, van Horssen J (2011) The molecular basis of neurodegeneration in multiple sclerosis. FEBS Lett 585:3715–3723
Lechner O, Dietrich H, OliveiradosSantos A, Wiegers GJ, Schwarz S, Harbutz M (2000) Altered circadian rhythms of the stress hormone and melatonin response in lupus-prone MRL/MP-fas(Ipr) mice. J Autoimmun 14:325–33
Lee MY, Kuan YH, Chen HY, Chen TY, Chen ST, Huang CC, Yang IP, Hsu YS, Wu TS, Lee EJ (2007) Intravenous administration of melatonin reduces the intracerebral cellular inflammatory response following transient focal cerebral ischemia in rats. J Pineal Res 42:297–309
Lee K, Choi GH, Back K (2017) Cadmium-induced melatonin synthesis in rice requires light, hydrogen peroxide, and nitric oxide, key regulatory roles for tryptophan decarboxylase and caffeic acid O-methyltransferase. J Pineal Res 63:e12441
Lenz SP, Izui S, Benediktsson H, Hart DA (1995) Lithium chloride enhances survival of NZB/W lupus mice, influence of melatonin and timing of treatment. Int J Immunopharmacol 17:581–592
Li PK, Witt-Enderby PA (2000) Melatonin receptors as potential targets for drug discovery. Drugs Future 25:945–957
Li DY, Smith DG, Hardeland R (2013) Melatonin receptor genes in vertebrates. Int J Mol Sci 14:11208–11223
Li Y, Li S, Zhou Y, Meng X, Zhang JJ, Xu DP, Li HB (2017) Melatonin for the prevention and treatment of cancer. Oncotarget 8:39896
Liao Z, Zhang X, Zhang Y, Peng D (2019) Seasonality and trend forecasting of tuberculosis incidence in Chongqing, China. Interdiscip Sci Comput Life Sci 11:77–85
Lin GJ, Huang SH, Chen YW, Hueng DY, Chien MW, Chia WT, Chang DM, Sytwu HK (2009) Melatonin prolongs islet graft survival in diabetic NOD mice. J Pineal Res 47:284–292
Liu C, Weaver DR, Jin X, Shearman LP, Pieschl RL, Gribkoff VK (1997) Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron 19:91–102
Liu J, Clough SJ, Hutchinson AJ, Adamah-Biassi EB, Popovska-Gorevski M, Dubocovich ML (2016) MT1 and MT2 melatonin receptors, a therapeutic perspective. Annu Rev Pharmacol Toxicol 56:361–383
Lopez-Gonzalez MA, Guerrero JM, Sanchez B, Delgado F (1997) Melatonin induces hyporeactivity caused by type II collagen in peripheral blood lymphocytes from patients with autoimmune hearing losses. Neurosci Lett 12(239):1–4
Lotufo CM, Lopes C, Dubocovich ML, Farsky SH, Markus RP (2001) Melatonin and N-acetylserotonin inhibit leukocyte rolling and adhesion to rat microcirculation. Eur J Pharmacol 2(430):351–357
Luboshitzky R, Shen-Orr Z, Herer P, Nave R (2003) Urinary 6-sulfatoxymelatonin excretion in hyperandrogenic women with polycystic ovary syndrome: the effect of ethinyl estradiol-cyproterone acetate treatment. Gynecol Endocrinol 17:441–447
Luboshitzky R, Herer P, Shen-Orr Z (2004) Urinary 6-sulfatoxymelatonin excretion in hyperandrogenic women: the effect of cyproterone acetate-ethinyl estradiol treatment. Exp Clin Endocrinol Diabetes 112:102–107
Ma C, Liu Y, Neumann S, Gao X (2017) Nicotine from cigarette smoking and diet and Parkinson disease: a review. Tranl Neurodegener 1:1–7
Maestroni GJ, Sulli A, Pizzorni C, Villaggio B, Cutolo M (2002) Melatonin in rheumatoid arthritis: synovial macrophages show melatonin receptors. Ann N Y Acad Sci 966:271–275
Maguire-Zeiss KA, Federoff HJ (2010) Future directions for immune modulation in neurodegenerative disorders: focus on Parkinson’s disease. J Neural Transm 117:1019–1025
Majidinia M, Sadeghpour A, Mehrzadi S, Reiter RJ, Khatami N, Yousefi B (2017) Melatonin: a pleiotropic molecule that modulates DNA damage response and repair pathways. J Pineal Res 63:12416
Maldonado MD, Moreno H, Calvo JR (2009) Melatonin present in beer contributes to increase the levels of melatonin and antioxidant capacity of the human serum. Clin Nutr 28:188–191
Mallaupoma LRC, Dias BKDM, Singh MK, Honorio RI, Nakabashi M, Kisukuri CDM, Paixão MW, Garcia CR (2022) Decoding the role of melatonin structure on Plasmodium falciparum human malaria parasites synchronization using 2-sulfenylindoles derivatives. Biomolecules 12:638
Manchester LC, Tan DX, Reiter RJ, Park W, Monis K, Qi W (2000) High levels of melatonin in the seeds of edible plants: possible function in germ tissue protection. Life Sci 67:3023–3029
Manchester LC, Coto-Montes A, Boga JA, Andersen LP, Zhou Z, Galano A, Vriend J, Tan DX, Reiter RJ (2015) Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J Pineal Res 9:403–19
Mansour HA, Wood J, Chowdari KV, Dayal M, Thase ME, Kupfer DJ, Monk TH, Devlin B, Nimgaonkar VL (2005) Circadian phase variation in bipolar I disorder. Chronobiol Int 1(2):571–584
Mantovani M, Kaster MP, Pertile R, Calixto JB, Rodrigues AL, Santos AR (2006) Mechanisms involved in the antinociception caused by melatonin in mice. J Pineal Res 41:382–389
Margheri M, Pacini N, Tani A, Nosi D, Squecco R, Dama A, Masala E, Francini F, Zecchi-Orlandini S, Formigli L (2012) Combined effects of melatonin and all-trans retinoic acid and somatostatin on breast cancer cell proliferation and death: molecular basis for the anticancer effect of these molecules. Eur J Pharmacol 68:34–43
Martin V, Herrera F, GarcíaSantos G, Antolín I, Rodriguez-Blanco J, Medina M, Rodriguez C (2007) Involvement of protein kinase C in melatonin’s oncostatic effect in C6 glioma cells. J Pineal Res 43:239–44
Masana MI, Dubocovich ML (2001). Melatonin receptor signaling: finding the path through the dark. Sci STKE 2001(107):pe39-pe39
Mason IC, Qian J, Adler GK, Scheer FA (2020) Impact of circadian disruption on glucose metabolism: implications for type 2 diabetes. Diabetologia 63:462–472
Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V, Rodriguez C (2002) Melatonin regulation of antioxidant enzyme gene expression. Cell Mol Life Sci 59:1706–1713
McInnes IB, Schett G (2011) The pathogenesis of rheumatoid arthritis. N Eng J Med 365:2205–2219
Mena P, Gil-Izquierdo Á, Moreno DA, Martí N, García-Viguera C (2012) Assessment of the melatonin production in pomegranate wines. LWT 47:13–8
Meyer RA (2006) Peripheral mechanisms of cutaneous nociception. Wall and Melzack's textbook of pain
Molinari EJ, North PC, Dubocovich ML (1996) 2-[125I] iodo-5-methoxycarbonylamino-N-acetyltryptamine: a selective radioligand for the characterization of melatonin ML2 binding sites. Eur J Pharmacol 301:159–168
Montiel M, Bonilla E, Valero N, Mosquera J, Espina LM, Quiroz Y, Álvarez-Mon M (2015) Melatonin decreases brain apoptosis, oxidative stress, and CD200 expression and increased survival rate in mice infected by Venezuelan equine encephalitis virus. Antivir Chem Chemother 24:99–108
Morgan PJ, Barrett P, Howell HE, Helliwell R (1994) Melatonin receptors: localization, molecular pharmacology and physiological significance. Neurochem Int 24:101–146
Mozzanica N, Tadini G, Radaelli A, Negri M, Pigatto P, Morelli M, Frigerio U, Finzi A, Esposti G, Rossi D (1988) Plasma melatonin levels in psoriasis. Acta Derm Venereol 68:312–316
Nakano Y, Oshima T, Ozono R, Higashi Y, Sasaki S, Matsumoto T, Matsuura H, Chayama K, Kambe M (2001) Non-dipper phenomenon in essential hypertension is related to blunted nocturnal rise and fall of sympatho-vagal nervous activity and progress in retinopathy. Auton Neurosci 88:181–186
Nava M, Quiroz Y, Vaziri N, Rodríguez-Iturbe B (2003) Melatonin reduces renal interstitial inflammation and improves hypertension in spontaneously hypertensive rats. Am J Physiol Ren Physiol 284:447–54
Nesi G, Sestito S, Digiacomo M, Rapposelli S (2017) Oxidative stress, mitochondrial abnormalities and proteins deposition: multitarget approaches in Alzheimer’s disease. Curr Top Med Chem 17:3062–3079
Nooshinfar E, Safaroghli-Azar A, Bashash D, Akbari ME (2017) Melatonin, an inhibitory agent in breast cancer. Breast Cancer 24:42–51
Nosjean O, Ferro M, Cogé F, Beauverger P, Henlin JM, Lefoulon F, Fauchere JL, Delagrange P, Canet E, Boutin JA (2000) Identification of the melatonin-binding siteMT 3 as the quinone reductase 2. J Biol Chem 275:31311–7
Olcese JM, Cao C, Mori T, Mamcarz MB, Maxwell A, Runfeldt MJ, Wang L, Zhang C, Lin X, Zhang G, Arendash GW (2009) Protection against cognitive deficits and markers of neurodegeneration by long term oral administration of melatonin in a transgenic model of Alzheimer disease. J Pineal Res 47:82–96
Oxenkrug GF, Summergrad P (2010) Ramelteon attenuates age associated hypertension and weight gain in spontaneously hypertensive rats. Ann N Y Acad Sci 199:114–120
Ozkank E, Yaman H, Cakir E, Deniz O, Oztosun M, Gumus S, Akgul EO, Agilli M, Cayci T, Kurt YG, Aydin I (2012) Plasma melatonin and urinary 6-hydroxymelatonin levels in patients with pulmonary tuberculosis. Inflammation 35:1429–1434
Padumanonda T, Johns J, Sangkasat A, Tiyaworanant S (2014) Determination of melatonin content in traditional Thai herbal remedies used as sleeping aids. DARU J Pharm Sci 22:1–6
Padurariu M, Ciobica A, Lefter R, Lacramioara-Serban I, Tefanescu C, Chirita R (2013) The oxidative stress hypothesis in Alzheimer’s disease. Psychiatr Danub 25:401–409
Pai SA, Majumdar AS (2014) Protective effects of melatonin against metabolic and reproductive disturbances in polycystic ovary syndrome in rats. J Pharm Pharmacol 66:1710–1721
Pandi-Perumal SR, Trakht I, Srinivasan V, Spence DW, Maestroni GJ, Zisapel N, Cardinali DP (2008) Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog Neurol 85:335–53
Pang SF, Dubocovich ML, Brown GM (1993) Melatonin receptors in peripheral tissues: a new area of melatonin research. Neurosignals 2:177–180
Pang CS, Tang PL, Song Y, Brown GM, Panga SF (1996) 2-[125I] Iodomelatonin binding sites in the quail heart: characteristics, distribution and modulation by guanine nucleotides and cations. Life Sci 58:1047–1057
Pardridge WM, Mietus LJ (1980) Transport of albumin-bound melatonin through the blood–brain barrier. J Neurochem 34:1761–1763
Paredes SD, Rancan L, Kireev R, González A, Louzao P, González P, Rodríguez-Bobada C, García C, Vara E, Tresguerres JA (2015) Melatonin counteracts at a transcriptional level the inflammatory and apoptotic response secondary to ischemic brain injury induced by middle cerebral artery blockade in aging rats. BioRes Open Acess 4:407–16
Patiño P, Parada E, Farré-Alins V, Molz S, Cacabelos R, Marco-Contelles J, López MG, Tasca CI, Ramos E, Romero A, Egea J (2016) Melatonin protects against oxygen and glucose deprivation by decreasing extracellular glutamate and Nox-derived ROS in rat hippocampal slices. Neurotoxicology 1(57):61–8
Paulis L (2006) The antihypertensive action of melatonin in spontaneously hypertensive rats: comparison with spironolactone. Hypertension 48:776
Pechanova O, Matuskova J, Capikova D, Jendekova L, Paulis L, Simko F (2006) Effect of spironolactone and captopril on nitric oxide and S-nitrosothiol formation in kidney of L-NAME-treated rats. Kid Int 1(70):170–176
Pei Z, Pang SF, Cheung RT (2003) Administration of melatonin after onset of ischemia reduces the volume of cerebral infarction in a rat middle cerebral artery occlusion stroke model. Stroke 34:770–775
Peschke E, Bähr I, Mühlbauer E (2013) Melatonin and pancreatic islets: interrelationships between melatonin, insulin and glucagon. Int J Mol Sci 14:6981–7015
Pinsky MR (2001) Sepsis: a pro- and anti-inflammatory disequilibrium syndrome. Contrib Nephrol 1(132):354–366
Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX (2006) Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 113:898–918
Prasad KN (2017) Oxidative stress and pro-inflammatory cytokines may act as one of the signals for regulating microRNAs expression in Alzheimer’s disease. Mech Ageing Dev 1(162):63–71
Prokopenko I, Langenberg C, Florez JC, Saxena R, Soranzo N, Thorleifsson G, Loos RJ, Manning AK, Jackson AU, Aulchenko Y, Potter SC (2009) Variants in MTNR1B influence fasting glucose levels. Nat Genet 41:77–81
Prunet-Marcassus B, Desbazeille M, Bros A, Louche K, Delagrange P, Renard P, Casteilla L, Pénicaud L (2003) Melatonin reduces body weight gain in Sprague Dawley rats with diet-induced obesity. Endocrinology 144:5347–52
Pshenichnyuk SA, Modelli A, Jones D, Lazneva EF, Komolov AS (2017) Low-energy electron interaction with melatonin and related compounds. J Phys Chem B 121:3965–3974
Pulimeno P, Mannic T, Sage D, Giovannoni L, Salmon P, Lemeille S, Giry-Laterriere M, Unser M, Bosco D, Bauer C, Morf J (2013) Autonomous and self-sustained circadian oscillators displayed in human islet cells. Diabetologia 56:497–507
Qin X, Zhang Y, Cai Y, He M, Sun L, Fu J, Li J, Wang B, Xing H, Tang G, Wang X (2013) Prevalence of obesity, abdominal obesity and associated factors in hypertensive adults aged 45–75 years. Clin Nutr 1(32):361–367
Ram PT, Dai J, Yuan L, Dong C, Kiefer TL, Lai L, Hill SM (2002) Involvement of the MT1 melatonin receptor in human breast cancer. Cancer Lett 28(179):141–150
Regodón S, Martín-Palomino P, Fernández-Montesinos R, Herrera JL, Carrascosa-Salmoral MP, Píriz S, Vadillo S, Guerrero JM, Pozo D (2005) The use of melatonin as a vaccine agent. Vaccine 23:5321–7
Regodón S, Ramos A, Míguez MP, Carrillo-Vico A, Rosado JA, Jardín I (2012) Vaccination prepartum enhances the beneficial effects of melatonin on the immune response and reduces platelet responsiveness in sheep. BMC Vet Res 8:1–8
Reiter RJ (1993) The melatonin rhythm: both a clock and a calendar. Experientia 49:654–664
Reiter RJ (2003) Melatonin: clinical relevance. Best Pract Res Clin Endocrinol Metab 1(17):273–285
Reiter RJ, Manchester LC, Tan DX (2005) Melatonin in walnuts: influence on levels of melatonin and total antioxidant capacity of blood. Nutrition 1(21):920–924
Reiter RJ, Tan DX, Manchester LC, Paredes SD, Mayo JC, Sainz RM (2009) Melatonin and reproduction revisited. Biol Reprod 81:445–456
Reiter RJ, Tan DX, Paredes SD, Fuentes-Broto L (2010) Beneficial effects of melatonin in cardiovascular disease. Ann Med 42:276–85
Reiter RJ, Tan DX, Galano A (2014) Melatonin: exceeding expectations. Physiology. https://doi.org/10.1152/physiol.00011.2014
Reiter RJ, Rosales-Corral S, Tan DX, Jou MJ, Galano A, Xu B (2017) Melatonin as a mitochondria-targeted antioxidant: one of evolution’s best ideas. Cell Mol Life Sci 74:3863–81
Reppert SM, Godson C, Mahle CD, Weaver DR, Slaugenhaupt SA, Gusella J (1995) Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc Natl Acad Sci USA 12(92):8734–8738
Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, Reiter RJ (2004) Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res 36:1–9
Rouillon F (2006) Efficacy and tolerance profile of agomelatine and practical use in depressed patients. Int Clin Psychopharmacol 1(21):S31–S35
Sánchez-Sánchez AM, Martín V, García-Santos G, Rodriguez-Blanco J, Casado-Zapico S, Suarez-Garnacho S, Antolin I, Rodriguez C (2011) Intracellular redox state as determinant for melatonin antiproliferative vs cytotoxic effects in cancer cells. Free Rad Res 1(45):1333–41
Sandyk R, Awerbuch GI (1992) Nocturnal plasma melatonin and alpha-melanocyte stimulating hormone levels during exacerbation of multiple sclerosis. Int J Neurosci 1(67):173–186
Sapmaz E, Ayar A, Celik H, Sapmaz T, Kilic N, Yasar MA (2003) Effects of melatonin and oxytetracycline in autologous intraperitoneal ovary transplantation in rats. Neuro Endocrinol Lett 1(24):350–354
Sato S (2021) Plasmodium—a brief introduction to the parasites causing human malaria and their basic biology. J Physiol Anthropol 40:1–13
Scheer FAJL, Van Montfrans GA, van Someren EJW, Mairuhu G, Buijs RM (2004) Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension 43:192–7
Shaikh N, Dadachanji R, Mukherjee S (2014) Genetic markers of polycystic ovary syndrome: emphasis on insulin resistance. Int J Med Genet 2014:1–10
Shavali S, Ho B, Govitrapong P, Sawlom S, Ajjimaporn A, Klongpanichapak S, Ebadi M (2005) Melatonin exerts its analgesic actions not by binding to opioid receptor subtypes but by increasing the release of β-endorphin an endogenous opioid. Brain Res Bull 30(64):471–479
Shieh JM, Wu HT, Cheng KC, Cheng JT (2009) Melatonin ameliorates high fat diet-induced diabetes and stimulates glycogen synthesis via a PKCζ-Akt-GSK3β pathway in hepatic cells. J Pineal Res 47:339–44
Shukla M, Govitrapong P, Boontem P, Reiter RJ, Satayavivad J (2017) Mechanisms of melatonin in alleviating Alzheimer’s disease. Curr Neuropharmacol 1(15):1010–1031
Simko F, Paulis L (2007) Melatonin as a potential antihypertensive treatment. J Pineal Res 42:319–322
Simko F, Pechanova O (2009) Potential roles of melatonin and chronotherapy among the new trends in hypertension treatment. J Pineal Res 47:127–133
Simonneaux V, Ribelayga C (2003) Generation of the melatonin endocrine message in mammals: a review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters. Pharmacol Rev 55:325–395
Singh B, Daneshvar C (2013) Human infections and detection of Plasmodium knowlesi. Clin Microbiol Rev 26:165–184
Sinha K, Degaonkar MN, Jagannathan NR, Gupta YK (2001) Effect of melatonin on ischemia reperfusion injury induced by middle cerebral artery occlusion in rats. Eur J Pharmacol 5(428):185–192
Skaper SD, Floreani M, Ceccon M, Facci L, Giusti P (1999) Excitotoxicity, oxidative stress, and the neuroprotective potential of melatonin. Ann N Y Acad Sci 890:107–118
Soliman MF, El Shenawy NS, El Arabi SE (2008) Schistosoma mansoni: melatonin enhances efficacy of cercarial and soluble worm antigens in the induction of protective immunity against infection in the hamster. Exp Parasitol 1(119):291–5
Sriskandan S, Cohen J (1995) The pathogenesis of septic shock. J Infect 1(30):201–206
Sturtz M, Cerezo AB, Cantos-Villar E, Garcia-Parrilla MC (2011) Determination of the melatonin content of different varieties of tomatoes (Lycopersicon esculentum) and strawberries (Fragaria ananassa). Food Chem 127:132934
Stys PK (1998) Anoxic and ischemic injury of myelinated axons in CNS white matter: from mechanistic concepts to therapeutics. J Cereb Blood Flow Metab 18:2–5
Subramanian P, Mirunalini S, Dakshayani KB, Pandi-Perumal SR, Trakht I, Cardinali DP (2007) Prevention by melatonin of hepatocarcinogenesis in rats injected with N-nitrosodiethylamine. J Pineal Res 43:305–12
Sulli A, Maestroni GJ, Villaggio B, Hertens E, Craviotto C, Pizzorni C, Briata M, Seriolo B, Cutolo M (2002) Melatonin serum levels in rheumatoid arthritis. Ann N Y Acad Sci 966:276–83
Sultana R, Butterfield DA (2010) Role of oxidative stress in the progression of Alzheimer’s disease. J Alzheimers Dis 1(19):341–353
Tamtaji OR, Mobini M, Reiter RJ, Azami A, Gholami MS, Asemi Z (2018) Melatonin, a toll-like receptor inhibitor: current status and future perspectives. J Cell Physiol 234:7788–7795
Tan DX, Manchester LC, Hardeland R, Lopez-Burillo S, Mayo JC, Sainz RM et al (2003) Melatonin: a hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J Pineal Res 34:75–78
Tan DX, Hardeland R, Manchester LC, Paredes SD, Korkmaz A, Sainz RM (2010) The changing biological roles of melatonin during evolution, from an antioxidant to signals of darkness, sexual selection and fitness. Biol Rev 85:607–623
Tan DX, Hardeland R, Manchester LC, Korkmaz A, Ma S, Rosales-Corral S, Reiter RJ (2012) Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J Exp Bot 1(63):577–97
Tan DX, Manchester LC, Esteban-Zubero E, Zhou Z, Reiter R (2015) Melatonin as a potent and inducible endogenous antioxidant, synthesis and metabolism. Molecules 20:18886
Tan DX, Hardeland R, Back K, Manchester LC, Alatorre-Jimenez MA, Reiter RJ (2016) On the significance of an alternate pathway of melatonin synthesisvia 5-methoxytryptamine, comparisons across species. J Pineal Res 61:27–40
Tang Y, Cai B, Yuan F, He X, Lin X, Wang J, Wang Y, Yang GY (2014) Melatonin pretreatment improves the survival and function of transplanted mesenchymal stem cells after focal cerebral ischemia. Cell Transplant 23:1279–1291
Tansey MG, McCoy MK, Frank-Cannon TC (2007) Neuroinflammatory mechanisms in Parkinson’s disease: potential environmental triggers, pathways, and targets for early therapeutic intervention. Exp Neurol 1(208):1–25
Tappe-Theodor A, Kuner R (2014) Studying ongoing and spontaneous pain in rodents—challenges and opportunities. Eur J Neurosci 39:1881–1890
Thiéblot L, Le Bars H (1955) La glande pinéale ou épiphyse: anatomie, histologie, physiologie, clinique. Maloine, Paris
Tordjman S, Chokron S, Delorme R, Charrier A, Bellissant E, Jaafari N, Fougerou C (2017) Melatonin: pharmacology, functions and therapeutic benefits. Curr Neuropharmacol 1(15):434–443
Torre LA, Lindsey A, Rebecca L, SiegeL RL, JemAL A (2016) Lung cancer statistics. Adv Exp Med Biol 893:1–9
Tsiotou AG, Sakorafas GH, Anagnostopoulos G, Bramis J (2005) Septic shock; current pathogenetic concepts from a clinical perspective. Med Sci Monit Intl Med J Exp Clin Res 1(11):76–85
Tsokos GC (2011) Systemic lupus erythematosus. N Engl J Med 365:2110–2121
Uz T, Arslan AD, Kurtuncu M, Imbesi M, Akhisaroglu M, Dwivedi Y, Pandey GN, Manev H (2005) The regional and cellular expression profile of the melatonin receptor MT1 in the central dopaminergic system. Mol Brain Res 20(136):45–53
van der Mei IA, Ponsonby AL, Dwyer T, Blizzard L, Simmons R, Taylor BV, Butzkueven H, Kilpatrick T (2003) Past exposure to sun, skin phenotype, and risk of multiple sclerosis: case–control study. BMJ 7(327):316
Vecchierini MF, Kilic-Huck U, Quera-Salva MA (2021) Melatonin (MEL) and its use in neurological diseases and insomnia: recommendations of the French Medical and Research Sleep Society (SFRMS). Rev Neurol 177:245–259
Venegas C, García JA, Escames G, Ortiz F, López A, Doerrier C, García-Corzo L, López LC, Reiter RJ, Acuña CD (2012) Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J Pineal Res 52:217–27
Victor VM, Rocha M, De la Fuente M (2004) Immune cells: free radicals and antioxidants in sepsis. Int Immunopharmacol 1(4):327–347
Von Gall C, Stehle JH, Weaver DR (2002) Mammalian melatonin receptors: molecular biology and signal transduction. Cell Tissue Res 309:151–62
Waldhauser F, Ehrhart B, Förster E (1993) Clinical aspects of the melatonin action: impact of development, aging, and puberty, involvement of melatonin in psychiatric disease and importance of neuroimmunoendocrine interactions. Experientia 49:671–681
Wang J, Xiao X, Zhang Y, Shi D, Chen W, Fu L, Liu L, Xie F, Kang T, Huang W, Deng W (2012) Simultaneous modulation of COX-2, p300, Akt, and Apaf-1 signaling by melatonin to inhibit proliferation and induce apoptosis in breast cancer cells. J Pineal Res 53:77–90
Watson N, Diamandis T, Gonzales-Portillo C, Reyes S, Borlongan CV (2016) Melatonin as an antioxidant for stroke neuroprotection. Cell Transplant 25:883–91
Weaver SC, Salas R, Rico-Hesse R, Ludwig GV, Oberste MS, Boshell J, Tesh RB, VEE Study Group (1996) Re-emergence of epidemic Venezuelan equine encephalomyelitis in South America. Lancet 17(348):436–40
West SK, Oosthuizen JM (1992) Melatonin levels are decreased in rheumatoid arthritis. J Basic Clin Physiol Pharmacol 1(3):33–40
White N (2008) Plasmodium knowlesi: the fifth human malaria parasite. Clin Infect Dis 46:172–173
Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J (2011) Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur J Epidemiol 26:1–58
Wongchitrat P, Shukla M, Sharma R, Govitrapong P, Reiter RJ (2010) Role of melatonin on virus-induced neuropathogenesis—a concomitant therapeutic strategy to understand SARS-CoV-2 infection. Antioxidants 10:47
Wu CC, Lu KC, Lin GJ, Hsieh HY, Chu P, Lin SH, Sytwu HK (2012) Melatonin enhances endogenous heme oxygenase-1 and represses immune responses to ameliorate experimental murine membranous nephropathy. J Pineal Res 52:460–469
Wu HJ, Wu C, Niu HJ, Wang K, Mo LJ, Shao AW, Dixon BJ, Zhang JM, Yang SX, Wang YR (2017) Neuroprotective mechanisms of melatonin in hemorrhagic stroke. Cell Mol Neurobiol 37:1173–1185
Wurtman RJ, Axelrod J, Chu EW (1963) Melatonin, a pineal substance: effect on the rat ovary. Science 141:277–278
Yaggi HK, Araujo AB, McKinlay JB (2006) Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care 1(29):657–661
Yano S, Moseley K, Fu X, Azen C (2016) Evaluation of tetrahydrobiopterin therapy with large neutral amino acid supplementation in phenylketonuria: effects on potential peripheral biomarkers, melatonin and dopamine, for brain monoamine neurotransmitters. PLoS ONE 11:e0160892
Yusof R, Lau YL, Mahmud R, Fong MY, Jelip J, Ngian HU, Mustakim S, Mat Hussin H, Marzuki N, Mohd Ali M (2014) High proportion of knowlesi malaria in recent malaria cases in Malaysia. Malar J 13:1–9
Zammit G, Schwartz H, Roth T, Wang-Weigand S, Sainati S, Zhang J (2009) The effects of ramelteon in a first-night model of transient insomnia. Sleep Med 1(10):55–59
Zawilska JB, Skene DJ, Arendt J (2009) Physiology and pharmacology of melatonin in relation to biological rhythms. Pharmacol Rep 61:383–410
Zeman M, Dulkova K, Bada V, Herichova I (2005) Plasma melatonin concentrations in hypertensive patients with the dipping and non-dipping blood pressure profile. Life Sci 76:1795–1803
Zhou LL, Wei W, Si JF, Yuan DP (2010) Regulatory effect of melatonin on cytokine disturbances in the pristane-induced lupus mice. Mediat Inflamm. https://doi.org/10.1155/2010/951210
Acknowledgements
The corresponding author acknowledges all the authors for their contribution.
Funding
No source of funding is associated with this article.
Author information
Authors and Affiliations
Contributions
AA, SBA, MB, MUR, ABW, SMR, and RRB contributed in the writing, editing, preparation of tables/figures, and drafting of the manuscript. All authors confirmed the final version for submission.
Corresponding author
Ethics declarations
Competing Interests
All the authors declare no competing interests.
Ethical Approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmad, S.B., Ali, A., Bilal, M. et al. Melatonin and Health: Insights of Melatonin Action, Biological Functions, and Associated Disorders. Cell Mol Neurobiol 43, 2437–2458 (2023). https://doi.org/10.1007/s10571-023-01324-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-023-01324-w