Abstract
Purpose of the Review
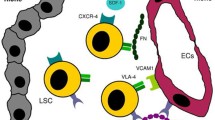
Herein we dissect mechanisms behind the dissemination of cancer cells from primary tumor site to the bone marrow, which are necessary for metastasis development, with a specific focus on multiple myeloma.
Recent Findings
The ability of tumor cells to invade vessels and reach the systemic circulation is a fundamental process for metastasis development; however, the interaction between clonal cells and the surrounding microenvironment is equally important for supporting colonization, survival, and growth in the secondary sites of dissemination. The intrinsic propensity of tumor cells to recognize a favorable milieu where to establish secondary growth is the basis of the “seed and soil” theory. This theory assumes that certain tumor cells (the “seeds”) have a specific affinity for the milieu of certain organs (the “soil”). Recent literature has highlighted the important contributions of the vascular niche to the hospitable “soil” within the bone marrow.
Summary
In this review, we discuss the crucial role of stromal cells and endothelial cells in supporting primary growth, homing, and metastasis to the bone marrow, in the context of multiple myeloma, a plasma cell malignancy with the unique propensity to primarily grow and metastasize to the bone marrow.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Croucher PI, McDonald MM, Martin TJ. Bone metastasis: the importance of the neighbourhood. Nat Rev Cancer. 2016;16(6):373–86. Comprehensive and detailed review describing pathophysiology of bone marrow metastasis with particular focus on the roles played by the cells of bone marrow microenvironment.
McDonald MM, et al. Adipose, bone, and myeloma: contributions from the microenvironment. Calcif Tissue Int. 2017;100(5):433–48.
Lawson MA, et al. Osteoclasts control reactivation of dormant myeloma cells by remodelling the endosteal niche. Nat Commun. 2015;6:8983.
Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011;11(6):411–25.
• Azab AK, et al. Hypoxia promotes dissemination of multiple myeloma through acquisition of epithelial to mesenchymal transition-like features. Blood. 2012;119(24):5782–94. First demonstration that multiple myeloma cells can behave as cancer epithelial cells in hypoxic conditions by undertaking the epithelial-to-mesenchymal transition leading to bone marrow-to-bone marrow metastasis.
Kingsley LA, et al. Molecular biology of bone metastasis. Mol Cancer Ther. 2007;6(10):2609–17.
•• Ghobrial IM. Myeloma as a model for the process of metastasis: implications for therapy. Blood. 2012;120(1):20–30. Here, multiple myeloma is described in details and for the first time as an ideal model of bone marrow-to-bone marrow metastasis which could be useful to study pathophysiology and biological mechanisms of bone metastasis.
Kawano Y, et al. Targeting the bone marrow microenvironment in multiple myeloma. Immunol Rev. 2015;263(1):160–72.
Krebsbach PH, et al. Bone marrow stromal cells: characterization and clinical application. Crit Rev Oral Biol Med. 1999;10(2):165–81.
Reagan MR, Ghobrial IM. Multiple myeloma mesenchymal stem cells: characterization, origin, and tumor-promoting effects. Clin Cancer Res. 2012;18(2):342–9.
Glavey, S.V., et al. Proteomic characterization of human multiple myeloma bone marrow extracellular matrix. Leukemia. 2017 https://doi.org/10.1038/leu.2017.102.
Zdzisinska B, et al. Abnormal cytokine production by bone marrow stromal cells of multiple myeloma patients in response to RPMI8226 myeloma cells. Arch Immunol Ther Exp. 2008;56(3):207–21.
Hideshima T, et al. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7(8):585–98.
Mitsiades CS, et al. The role of the bone microenvironment in the pathophysiology and therapeutic management of multiple myeloma: interplay of growth factors, their receptors and stromal interactions. Eur J Cancer. 2006;42(11):1564–73.
Kawano M, et al. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988;332(6159):83–5.
Podar K, Chauhan D, Anderson KC. Bone marrow microenvironment and the identification of new targets for myeloma therapy. Leukemia. 2009;23(1):10–24.
Le Gouill S, et al. Mcl-1 regulation and its role in multiple myeloma. Cell Cycle. 2004;3(10):1259–62.
Tai YT, et al. Role of B-cell-activating factor in adhesion and growth of human multiple myeloma cells in the bone marrow microenvironment. Cancer Res. 2006;66(13):6675–82.
Giuliani N, et al. Angiogenesis and multiple myeloma. Cancer Microenviron. 2011;4(3):325–37.
Corre J, et al. Bone marrow mesenchymal stem cells are abnormal in multiple myeloma. Leukemia. 2007;21(5):1079–88.
Corre J, et al. Bioactivity and prognostic significance of growth differentiation factor GDF15 secreted by bone marrow mesenchymal stem cells in multiple myeloma. Cancer Res. 2012;72(6):1395–406.
Tanno T, et al. Growth differentiating factor 15 enhances the tumor-initiating and self-renewal potential of multiple myeloma cells. Blood. 2014;123(5):725–33.
Roccaro AM, et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest. 2013;123(4):1542–55.
• Azab AK, et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009;113(18):4341–51. First preclinical evidence that targeting the SDF1alpha/CXCR4 pathway can mobilize multiple myeloma cells in the circulation and render them sensitive to anti-myeloma treatment, implying that bone marrow homing supports drug resistance in multiple myeloma.
Weinstock M, Ghobrial IM. Extramedullary multiple myeloma. Leuk Lymphoma. 2013;54(6):1135–41.
•• Roccaro AM, et al. CXCR4 regulates extra-medullary myeloma through epithelial-mesenchymal-transition-like transcriptional activation. Cell Rep. 2015;12(4):622–35. Here for the first time, the SDF1 alpha/CXCR4 axis and the epithelial to mesenchymal transition process are described to be involved in the promotion of metastasis to solid organs (extramedullary disease) in multiple myeloma.
Keller S, et al. Exosomes: from biogenesis and secretion to biological function. Immunol Lett. 2006;107(2):102–8.
Wang J, et al. Bone marrow stromal cell-derived exosomes as communicators in drug resistance in multiple myeloma cells. Blood. 2014;124(4):555–66.
Kusumbe AP. Vascular niches for disseminated tumour cells in bone. J Bone Oncol. 2016;5(3):112–6.
Silberstein LE, Lin CP. A new image of the hematopoietic stem cell vascular niche. Cell Stem Cell. 2013;13(5):514–6.
Boulais PE, Frenette PS. Making sense of hematopoietic stem cell niches. Blood. 2015;125(17):2621–9.
Bussard KM, Gay CV, Mastro AM. The bone microenvironment in metastasis; what is special about bone? Cancer Metastasis Rev. 2008;27(1):41–55.
Ghajar CM. Metastasis prevention by targeting the dormant niche. Nat Rev Cancer. 2015;15(4):238–47.
Ghajar CM, et al. The perivascular niche regulates breast tumor dormancy. Nat Cell Biol. 2013;15(7):807–17.
Shiozawa Y, Pienta KJ, Taichman RS. Hematopoietic stem cell niche is a potential therapeutic target for bone metastatic tumors. Clin Cancer Res. 2011;17(17):5553–8.
Winkler IG, et al. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat Med. 2012;18(11):1651–7.
Glavey SV, et al. The sialyltransferase ST3GAL6 influences homing and survival in multiple myeloma. Blood. 2014;124(11):1765–76.
Glavey SV, et al. The cancer glycome: carbohydrates as mediators of metastasis. Blood Rev. 2015;29(4):269–79.
Folkman J, et al. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339(6219):58–61.
Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438(7070):932–6.
Vacca A, Ribatti D. Angiogenesis and vasculogenesis in multiple myeloma: role of inflammatory cells. Recent Results Cancer Res. 2011;183:87–95.
•• Vacca A, et al. Bone marrow of patients with active multiple myeloma: angiogenesis and plasma cell adhesion molecules LFA-1, VLA-4, LAM-1, and CD44. Am J Hematol. 1995;50(1):9–14. These studies for the first time identified the phenomenon of neo-angiogenesis within the bone marrow of patients with MM: specifically, the authors demonstrated an increased bone marrow microvascular density in active MM patients compared to those with inactive MM and MGUS.
Ribatti D, et al. Angiogenesis spectrum in the stroma of B-cell non-Hodgkin's lymphomas. An immunohistochemical and ultrastructural study. Eur J Haematol. 1996;56(1–2):45–53.
Rajkumar SV, et al. Bone marrow angiogenesis in 400 patients with monoclonal gammopathy of undetermined significance, multiple myeloma, and primary amyloidosis. Clin Cancer Res. 2002;8(7):2210–6.
Kumar S, et al. Bone marrow angiogenic ability and expression of angiogenic cytokines in myeloma: evidence favoring loss of marrow angiogenesis inhibitory activity with disease progression. Blood. 2004;104(4):1159–65.
Hose D, et al. Induction of angiogenesis by normal and malignant plasma cells. Blood. 2009;114(1):128–43.
Jakob C, et al. Angiogenesis in multiple myeloma. Eur J Cancer. 2006;42(11):1581–90.
Kumar S, et al. Prognostic value of bone marrow angiogenesis in patients with multiple myeloma undergoing high-dose therapy. Bone Marrow Transplant. 2004;34(3):235–9.
Kumar S, et al. Prognostic value of angiogenesis in solitary bone plasmacytoma. Blood. 2003;101(5):1715–7.
Kumar S, et al. Bone marrow angiogenesis in multiple myeloma: effect of therapy. Br J Haematol. 2002;119(3):665–71.
Kumar S, et al. Effect of thalidomide therapy on bone marrow angiogenesis in multiple myeloma. Leukemia. 2004;18(3):624–7.
Vacca A, et al. Bone marrow angiogenesis and progression in multiple myeloma. Br J Haematol. 1994;87(3):503–8.
Xu JL, et al. Proliferation, apoptosis, and intratumoral vascularity in multiple myeloma: correlation with the clinical stage and cytological grade. J Clin Pathol. 2002;55(7):530–4.
Kumar S, et al. Bone marrow angiogenesis and circulating plasma cells in multiple myeloma. Br J Haematol. 2003;122(2):272–4.
Nowakowski GS, et al. Circulating plasma cells detected by flow cytometry as a predictor of survival in 302 patients with newly diagnosed multiple myeloma. Blood. 2005;106(7):2276–9.
Kumar S, et al. Cell proliferation of myeloma plasma cells: comparison of the blood and marrow compartments. Am J Hematol. 2004;77(1):7–11.
Ribatti D, Mangialardi G, Vacca A. Antiangiogenic therapeutic approaches in multiple myeloma. Curr Cancer Drug Targets. 2012;12(7):768–75.
Schmidt T, Carmeliet P. Angiogenesis: a target in solid tumors, also in leukemia? Hematology Am Soc Hematol Educ Program. 2011;2011:1–8.
Holash J, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284(5422):1994–8.
Chang YS, et al. Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci U S A. 2000;97(26):14608–13.
Hendrix MJ, et al. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer. 2003;3(6):411–21.
Asahara T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–7.
de Bont ES, et al. Mobilized human CD34+ hematopoietic stem cells enhance tumor growth in a nonobese diabetic/severe combined immunodeficient mouse model of human non-Hodgkin’s lymphoma. Cancer Res. 2001;61(20):7654–9.
Pelosi E, et al. Identification of the hemangioblast in postnatal life. Blood. 2002;100(9):3203–8.
Dome B, et al. Alternative vascularization mechanisms in cancer: pathology and therapeutic implications. Am J Pathol. 2007;170(1):1–15.
Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8(8):592–603.
Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358(19):2039–49.
Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86(3):353–64.
Ribatti D, et al. The structure of the vascular network of tumors. Cancer Lett. 2007;248(1):18–23.
Moehler TM, et al. Angiogenesis in hematologic malignancies. Crit Rev Oncol Hematol. 2003;45(3):227–44.
Yang R, Han ZC. Angiogenesis in hematologic malignancies and its clinical implications. Int J Hematol. 2002;75(3):246–56.
Rajkumar SV, Mesa RA, Tefferi A. A review of angiogenesis and anti-angiogenic therapy in hematologic malignancies. J Hematother Stem Cell Res. 2002;11(1):33–47.
Ribatti D, et al. The history of the angiogenic switch concept. Leukemia. 2007;21(1):44–52.
Dome B, et al. Vascularization of cutaneous melanoma involves vessel co-option and has clinical significance. J Pathol. 2002;197(3):355–62.
Pezzella F, et al. Non-small-cell lung carcinoma tumor growth without morphological evidence of neo-angiogenesis. Am J Pathol. 1997;151(5):1417–23.
Maniotis AJ, et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155(3):739–52.
Streubel B, et al. Lymphoma-specific genetic aberrations in microvascular endothelial cells in B-cell lymphomas. N Engl J Med. 2004;351(3):250–9.
Rigolin GM, et al. Neoplastic circulating endothelial cells in multiple myeloma with 13q14 deletion. Blood. 2006;107(6):2531–5.
Gunsilius E, et al. Evidence from a leukaemia model for maintenance of vascular endothelium by bone-marrow-derived endothelial cells. Lancet. 2000;355(9216):1688–91.
Scavelli C, et al. Vasculogenic mimicry by bone marrow macrophages in patients with multiple myeloma. Oncogene. 2008;27(5):663–74.
Vacca A, et al. Bone marrow neovascularization, plasma cell angiogenic potential, and matrix metalloproteinase-2 secretion parallel progression of human multiple myeloma. Blood. 1999;93(9):3064–73.
Di Raimondo F, et al. Angiogenic factors in multiple myeloma: higher levels in bone marrow than in peripheral blood. Haematologica. 2000;85(8):800–5.
Nico B, et al. Mast cells contribute to vasculogenic mimicry in multiple myeloma. Stem Cells Dev. 2008;17(1):19–22.
Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91.
Moschetta M, et al. Role of endothelial progenitor cells in cancer progression. Biochim Biophys Acta. 2014;1846(1):26–39.
• Moschetta M, et al. Targeting vasculogenesis to prevent progression in multiple myeloma. Leukemia. 2016;30(5):1103–15. Here a new mouse model to study bone marrow to bone marrow metastasis in multiple myeloma is described; this model is used to demonstrate the recruitment of bone marrow-derived cells in other sites of bone marrow where multiple myeloma cells are localized.
Scavelli C, et al. Zoledronic acid affects over-angiogenic phenotype of endothelial cells in patients with multiple myeloma. Mol Cancer Ther. 2007;6(12 Pt 1):3256–62.
Acknowledgements
The authors would like to thank Associazione Italiana per la Ricerca sul Cancro (AIRC), Associazione Italiana contro le Leucemie, Linfomi e Mieloma (AIL)—Brescia, and European Hematology Association (EHA). Support was also provided by MMCRI Start-up funds, a pilot project grant from NIH/NIGMS (P30GM106391), and the NIH/NIDDK (R24DK092759-01) (for MRR).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Michele Moschetta, Yawara Kawano, Antonio Sacco, Angelo Belotti, Rossella Ribolla, Marco Chiarini, Viviana Giustini, Diego Bertoli, Alessandra Sottini, Monica Valotti, Claudia Ghidini, Federico Serana, Michele Malagola, Luisa Imberti, Domenico Russo, Alessandro Montanelli, Giuseppe Rossi, Michaela R. Reagan, Patricia Maiso, Bruno Paiva, Irene M. Ghobrial, and Aldo M. Roccaro declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Cancer-induced Musculoskeletal Diseases
Rights and permissions
About this article
Cite this article
Moschetta, M., Kawano, Y., Sacco, A. et al. Bone Marrow Stroma and Vascular Contributions to Myeloma Bone Homing. Curr Osteoporos Rep 15, 499–506 (2017). https://doi.org/10.1007/s11914-017-0399-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-017-0399-3