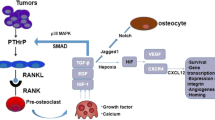
Abstract
Tumors such as breast, lung, and prostate frequently metastasize to bone, where they can cause intractable pain and increase the risk of fracture in patients. When tumor cells metastasize to bone, they interact with the microenvironment to promote bone destruction primarily through the secretion of osteolytic factors by the tumor cells and the subsequent release of growth factors from the bone. Our recent data suggest that the differential rigidity of the mineralized bone microenvironment relative to that of soft tissue regulates the expression of osteolytic factors by the tumor cells. The concept that matrix rigidity regulates tumor growth is well established in solid breast tumors, where increased rigidity stimulates tumor cell invasion and metastasis. Our studies have indicated that a transforming growth factor-β (TGF-β) and Rho-associated kinase (ROCK)–dependent mechanism is involved in the response of metastatic tumor cells to the rigid mineralized bone matrix. In this review, we will discuss the interactions between ROCK and TGF-β signaling, as well as potential new therapies that target these pathways.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Guise T, Yin J, Taylor S, Kumagai Y, Dallas M, Boyce B, et al. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Invest. 1996;98:1544–9.
Smith MR. Zoledronic acid to prevent skeletal complications in cancer: corroborating the evidence. Cancer Treat Rev. 2005;31 Suppl 3:19–25.
Stresing V, Daubine F, Benzaid I, Monkkonen H, Clezardin P. Bisphosphonates in cancer therapy. Cancer Lett. 2007;257:16–35.
Daubine F, Le Gall C, Gasser J, Green J, Clezardin P. Antitumor effects of clinical dosing regimens of bisphosphonates in experimental breast cancer bone metastasis. J Natl Cancer Inst. 2007;99:322–30.
Coleman RE, Lipton A, Roodman GD, Guise TA, Boyce BF, Brufsky AM, et al. Metastasis and bone loss: advancing treatment and prevention. Cancer Treat Rev. 2010;36:615–20.
Sterling JA, Edwards JR, Martin TJ, Mundy GR. Advances in the biology of bone metastasis: how the skeleton affects tumor behavior. Bone. 2011;48:6–15.
Yin JJ, Selander K, Chirgwin JM, Dallas M, Grubbs BG, Wieser R, et al. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest. 1999;103:197–206.
Southby J, Kissin MW, Danks JA, Hayman JA, Moseley JM, Henderson MA, et al. Immunohistochemical localization of parathyroid hormone-related protein in human breast cancer. Cancer Res. 1990;50:7710–6.
Powell GJ, Southby J, Danks JA, Stillwell RG, Hayman JA, Henderson MA, et al. Localization of parathyroid hormone-related protein in breast cancer metastases: increased incidence in bone compared with other sites. Cancer Res. 1991;51:3059061.
Henderson MA, Danks JA, Slavin JL, Byrnes GB, Choong PF, Spillane JB, et al. Parathyroid hormone-related protein localization in breast cancers predict improved prognosis. Cancer Res. 2006;66:2250–6.
Pirola CJ, Wang HM, Strgacich MI, Kamyar A, Cercek B, Forrester JS, et al. Mechanical stimuli induce vascular parathyroid hormone-related protein gene expression in vivo and in vitro. Endocrinology. 1994;134:2230–6.
Yamamoto M, Harm SC, Grasser WA, Thiede MA. Parathyroid hormone-related protein in the rat urinary bladder: a smooth muscle relaxant produced locally in response to mechanical stretch. Proc Natl Acad Sci USA. 1992;89:5326–30.
Philbrick WM, Wysolmerski JJ, Galbraith S, Holt E, Orloff JJ, Yang KH, et al. Defining the roles of parathyroid hormone-related protein in normal physiology. Physiol Rev. 1996;76:127–73.
Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–54.
Paszek MJ, Weaver VM. The tension mounts: mechanics meets morphogenesis and malignancy. J Mammary Gland Biol Neoplasia. 2004;9:325–42.
Geiger B, Bershaksky A. Exploring the neighborhood: adhesion-coupled cell mechanotransducers. Cell. 2002;110:139–43.
Galliher AJ, Schiemann WP. Beta3 integrin and Src facilitate transforming growth factor-beta mediated induction of epithelial-mesenchymal transition in mammary epithelial cells. Breast Cancer Res. 2006;8(4):R42.
Arias-Salgado EG, Lizano S, Sarkar S, Brugge JS, Ginsberg MH, Shattil SJ. Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc Natl Acad Sci USA. 2003;100:13298–302.
• Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer 2009, 9:108–122. This review highlights the role of mechanical forces in the invasion and progression of breast tumors.
Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22.
Galliher AJ, Schiemann WP. Src phosphorylates Tyr284 in TGF-beta type II receptor and regulates TGF-beta stimulation of p38 MAPK during breast cancer cell proliferation and invasion. Cancer Res. 2007;67:3752–8.
Schneider JG, Amend SR, Weilbaecher KN. Integrins and bone metastasis: integrating tumor cell and stromal cell interactions. Bone. 2011;48:54–65.
Moore SW, Roca-Cusachs P, Sheetz MP. Stretchy proteins on stretchy substrates: the important elements of integrin-mediated rigidity sensing. Dev Cell. 2010;19:194–206.
Kumar S, Weaver VM. Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev. 2009;28:113–27.
Bakin AV, Rinehart C, Tomlinson AK, Arteaga CL. p38 mitogen-activated protein kinase is required for TGFbeta-mediated fibroblastic transdifferentiation and cell migration. J Cell Sci. 2002;115:3193–206.
•• Khatiwala CB, Kim PD, Peyton SR, Putnam AJ. ECM compliance regulates osteogenesis by influencing MAPK signaling downstream of RhoA and ROCK. J Bone Miner Res 2009, 24:886–898. This study demonstrates that osteoblast differentiation is regulated by the rigidity of the extracellular matrix in bone through ROCK, and that blocking ROCK delays differentiation.
•• Liu S, Goldstein RH, Scepansky EM, Rosenblatt M. Inhibition of rho-associated kinase signaling prevents breast cancer metastasis to human bone. Cancer Res 2009, 69:8742–8751. This study demonstrates that molecular or pharmacologic inhibition of ROCK blocks metastasis of breast tumors to bone, and overexpression of ROCK confers a metastatic phenotype to a non-metastatic cell line. It is also suggested that inhibition of ROCK could represent a novel therapy for treatment of breast cancer metastases.
•• Kostic A, Lynch CD, Sheetz MP. Differential matrix rigidity response in breast cancer cell lines correlates with the tissue tropism. PLoS One 2009, 4:e6361. This study demonstrates that SCPs derived from MDA-MB-231 cells showed increased migration to proliferation when cultured on matrices with rigidities corresponding to the native rigidities of the organs where metastasis was observed. Thus, SCPs targeted specifically to bone proliferated faster and were more invasive on rigid tissue culture polystyrene compared with soft PAA gels.
•• Ruppender NS, Merkel AR, Martin TJ, Mundy GR, Sterling JA, Guelcher SA. Matrix Rigidity Induces Osteolytic Gene Expression of Metastatic Breast Cancer Cells. PLoS One 2010, 5:e15451. This study demonstrates that osteolytic breast and lung tumor cells cultured on substrates with rigidities comparable to that of mineralized bone tissue show increased expression of Gli2 and PTHrP compared with cells cultured on soft substrates. Furthermore, both ROCK and TGF-β are required for the increased PTHrP expression on rigid substrates.
Zaman MH, Trapani LM, Sieminski A, MacKellar D, Gong H, Kamm RD, et al. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. PNAS. 2006;103:10889–94.
Alexander NR, Branch KM, Iwueke IC, Guelcher SA, Weaver AM. Extracellular matrix rigidity promotes invadopodia activity. Curr Biol. 2008;18:1295–9.
Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89.
Guelcher SA, Dumas J, Srinivasan A, Didier JE, Hollinger JO. Synthesis, mechanical properties, biocompatibility, and biodegradation of polyurethane networks from lysine polyisocyanates. Biomaterials. 2008;29:1762–75.
Gibson LJ, Ashby MF. Cellular solids: structure and properties. Cambridge: Cambridge University Press; 1997.
Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordo’n-Cardo C, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–49.
Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–43.
Mundy GR. Mechanisms of bone metastasis. Cancer. 1997;80:1546–56.
Kakonen SM, Selander KS, Chirgwin JM, Yin JJ, Burns S, Rankin WA, et al. Transforming growth factor-beta stimulates parathyroid hormone-related protein and osteolytic metastases via Smad and mitogen-activated protein kinase signaling pathways. J Biol Chem. 2002;277:24571–8.
•• Kita T, Hata Y, Arita R, Kawahara S, Miura M, Nakao S, et al. Role of TGF-beta in proliferative vitreoretinal diseases and ROCK as a therapeutic target. Proc Natl Acad Sci U S A 2008, 105:17504–17509. This study elucidates the critical role of TGF-β in mediating cicatricial contraction in proliferative vitreoretinal diseases. ROCK, a key downstream mediator of TGF-β and other factors, might become a unique therapeutic target in the treatment of proliferative vitreoretinal diseases.
Kamaraju AK, Roberts AB. Role of Rho/ROCK and p38 MAP kinase pathways in transforming growth factor-beta-mediated Smad-dependent growth inhibition of human breast carcinoma cells in vivo. J Biol Chem. 2005;280:1024–36.
• Shane E. Evolving data about subtrochanteric fractures and bisphosphonates. N Engl J Med 2010 May 13, 362:1825–1827. Bisphosphonates, the major class of drugs used to treat osteoporosis, decrease osteoclast-mediated bone resorption and bone turnover markers and increase bone mineral density. They have been shown to be safe and reduce the risk of osteoporotic fractures.
McClung MR. Inhibition of RANKL as a treatment for osteoporosis: preclinical and early clinical studies. Curr Osteoporos Rep. 2006;4:28–33.
Lipton A, Steger GG, Figueroa J, Alvarado C, Solal-Celigny P, Body JJ, et al. Extended efficacy and safety of denosumab in breast cancer patients with bone metastases not receiving prior bisphosphonate therapy. Clin Cancer Res. 2008;14:6690–6.
Gonzalez-Suarez E, Jacob AP, Jones J, Miller R, Roudier-Meyer MP, Erwert R, et al. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature. 2010;468:103–7.
•• Ganapathy V, Ge R, Grazioli A, Xie W, Banach-Petrosky W, Kang Y, et al. Targeting the Transforming Growth Factor-beta pathway inhibits human basal-like breast cancer metastasis. Mol Cancer 2010, 9:122. This study shows that TGF-β plays an important role in both bone and lung metastases of breast cancer. Furthermore, inhibiting TGF-β signaling shows a therapeutic effect that is independent of the tissue tropism of the metastatic tumor cells. Targeting TGF-β signaling is a potentially useful therapeutic approach for treating metastatic basal-like breast cancer.
•• Mohammad KS, Javelaud D, Fournier PG, Niewolna M, McKenna CR, Peng XH, et al. The Transforming Growth Factor-{beta} Receptor I Kinase Inhibitor SD-208 Reduces the Development and Progression of Melanoma Bone Metastases. Cancer Res 2011, 71:175–84. This study demonstrates that therapeutic targeting of TGF-β may prevent the development of melanoma bone metastases and decrease the progression of established osteolytic lesions.
• Edwards JR, Nyman JS, Lwin ST, Moore MM, Esparza J, O'Quinn EC, et al. Inhibition of TGF-β signaling by 1D11 antibody treatment increases bone mass and quality in vivo. J Bone Miner Res 2010, 25:2419–2426. This study demonstrates that blocking TGF-β with 1D11 increases the population of osteoblasts and decreases the population of active osteoclasts in the marrow, significantly increasing bone volume and quality.
•• Tan AR, Alexe G, Reiss M. Transforming growth factor-beta signaling: emerging stem cell target in metastatic breast cancer? Breast Cancer Res Treat 2009, 115:453–495. This study demonstrates that in genetic mouse models in which TGF-β signaling is stimulated, TGF-β inhibits formation of mammary tumors at early stages, but can promote metastasis of tumors at later stages. These observations suggest that although TGF-β inhibitors may block bone resorption in patients with bone metastases, they could possibly increase tumor cell growth in the primary site.
•• Barman SA, Zhu S, White RE. RhoA/Rho-kinase signaling: a therapeutic target in pulmonary hypertension. Vasc Health Risk Manag 2009, 5:663–671. This paper reviews the extensive animal studies reporting that increased RhoA/ROCK signaling is important in the pathogenesis of pulmonary hypertension by stimulating enhanced construction and remodeling of the pulmonary vasculature. Both preclinical and clinical studies suggest that ROCK inhibitors are effective for treatment of severe pulmonary arterial hypertension (PAH) with minimal safety risk, which underscores the potential of ROCK inhibitors as a new class of drugs for treatment of PAH.
Olson MF. Applications for ROCK kinase inhibition. Curr Opin Cell Biol. 2008;20:242–8.
Acknowledgment
The authors acknowledge financial support from the National Institutes of Health Breast Cancer SPORE (P50 CA098131), P01 CA040035, and Tumor Microenvironment Network (TMEN) (U54 CA126505) grants.
Disclosure
Conflicts of interest: J.A. Sterling: none; S.A. Guelcher: has been a consultant and has received funding for his laboratory from Osteotech, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sterling, J.A., Guelcher, S.A. Bone Structural Components Regulating Sites of Tumor Metastasis. Curr Osteoporos Rep 9, 89–95 (2011). https://doi.org/10.1007/s11914-011-0052-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-011-0052-5