Abstract
Osteoporosis and several other bone disorders occur when there is an imbalance between the resorption and formation components of bone remodeling activity. Therapies available for some of these conditions modulate the activity of osteoclasts and/or osteoblasts. The recent discoveries of receptor activator of NF-кB ligand (RANKL), an endogenous activator of osteoclastogenenesis and osteoclast activity and its inhibitor, osteoprotegerin (OPG) as pivotal regulatory factors in the pathogenesis of bone diseases like osteoporosis provide unique targets for therapeutic agents. In laboratory animals and now in humans, administering forms of OPG markedly inhibits osteoclast activity and improves bone strength, documenting that the strategy of inhibiting RANKL activity has therapeutic promise. A highly specific, fully human antibody against RANKL has been produced (denosumab) that in early studies in humans reduces bone turnover and improves bone density. Attributes of denosumab in these clinical studies include a very rapid onset of action, sustained effects for several months after a single injection, and good tolerability. These results provide the basis for studies evaluating the effectiveness of denosumab in several clinical conditions characterized by increased osteoclastic activity.
Similar content being viewed by others
References and Recommended Reading
Chambers TJ: Regulation of the differentiation and function of osteoclasts. J Pathol 2000, 192:4–13.
Epker BN, Frost BN: Correlation of bone resorption and formation with the physical behaviour of loaded bone. J Dent Res 1965, 44:33–41.
Teitlebaum SL: Bone resorption by osteoclasts. Science 2000, 289:1504–1508.
Nicholson GC, Moseley JM, Sexton PM, et al.: Abundant calcitonin receptors in isolated osteoclasts. J Clin Invest 1986, 78:355–360.
Silve CH, Hradek GT, Jones AL, et al.: Parathyroid hormone receptor in intact embryonic chicken bone: characterization and cellular localization. J Cell Biol 1982, 94:39–386.
Jilka RL, Hangoc G, Girasole G, et al.: Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science 1992, 257:88–89.
Eriksen EF, Colvard DS, Berg NJ, et al.: Evidence of estrogen receptors in normal human osteoblast-like cells. Science 1988, 241:84–86.
Takahashi N, Akatsu T, Saaki T, et al.: Osteoblastic cells are involved in osteoclast formation. Endocrinology 1998, 123:2600–2602.
Simonet WS, Lacey DL, Dunstan CR, et al.: Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 1997, 89:309–319.
Tsuda E, Goto M, Michizuki S, et al.: Isolation of a novel cytokine from human fibroblasts that specifically inhibits osteoclastogenesis. Biochem Biophys Res Commun 1997, 234:137–142.
Anderson DM, Maraskovsky E, Billingsley WL, et al.: A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 1997, 390:175–179.
Wong BR, Josien R, Lee SY, et al.: TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J Exp Med 1997, 186:2075–2080.
ASBMR President’s Committee on Nomenclature. Proposed standard nomenclature for new tumor necrosis factor family members involved in the regulation of bone resorption. J Bone Miner Res 2000, 15:2293–2297.
Lacey DL, Tan HC, Lu J, et al.: Osteoprotegerin ligand modulates murine osteoclast survival in vitro and in vivo. Am J Pathol 2000, 157:435–448.
Bucay N, Sarosi I, Dunstan CR, et al.: Osteoprotegerinde ficient mice develop early onset osteoporosis and arterial calcification. Gene Dev 1998, 12:1260–1268.
Iotsova V, Caamano J, Loy J, et al.: Osteopetrosis in mice lacking NF-kappaB1 and NF-kappa B2. Nat Med 1997, 3:1285–1289.
Kong YY, Yoshida H, Sarosi I, et al.: OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 1999, 397:315–323.
Hof bauer LC, Dunstan CR, Spelsberg TC, et al.: Osteoprotegerin production by human osteoblast lineage cells is stimulated by vitamin D, bone morphogenetic protein-2, and cytokines. Biochem Biophys Res Commun 1998, 250:776–781.
Huang JC, Sakata T, Pfieger LL, et al.: PTH differentially regulates expression of RANKL and OPG. J Bone Miner Res 2004, 19:235–244.
Hof bauer LC, Lacey DL, Dunstan CR, et al.: Interleukin-1beta and tumor necrosis factor-alpha, but not interleukin-6, stimulate osteoprotegerin ligand gene expression in human osteoblastic cells. Bone 1999, 25:255–259.
Hof bauer LC, Khosla S, Dunstan CR, et al.: Estrogen stimulates gene expression and protein production of osteoprotegerin in human osteoblastic cells. Endocrinology 1999, 140:4367–4370.
Viereck V, Grundker C, Blaschke S, et al.: Raloxifene concurrently stimulates osteoprotegerin and inhibits interleukin-6 production by human trabecular osteoblasts. J Clin Endocrinol Metab 2003, 88:4206–4213.
Lochlin RM, Khosla S, Turner RT, Riggs BL: Mediators of the bisphasic responses of bone to intermittent and continuously administered parathyroid hormone. J Cell Biochem 2003, 89:180–190.
Hof bauer LC, Gori F, Riggs BL, et al.: Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells. Endocrinology 1999, 140:4382–4389.
Standal T, Seidel C, Hjertner O, et al.: Osteoprotegerin is bound, internalized, and degraded by multiple myeloma cells. Blood 2002, 100:3002–3007.
Okada T, Akikusa S, Okuno H, Kodaka M: Bone marrow metastatic myeloma cells promote osteoclastogenesis through RANKL on endothelial cells. Clin Exp Metastasis 2003, 20:639–646.
Johnson-Pais TL, Singer FR, Bone HG, et al.: Identification of a novel tandem duplication in exon 1 of the TNFRSF11A gene in two unrelated patients with familial expansile osteolysis. J Bone Miner Res 2003, 18:376–380.
Whyte MP, Hughes AE: Expansile skeletal hyperphosphatasia is caused by a 15-base pair tandem duplication in TNFRSF11A encoding RANK and is allelic to familial expansile osteolysis. J Bone Miner Res 2002, 17:26–29.
Whyte MP, Obrecht SE, Finnegan PM, et al.: Osteoprotegerin deficiency and juvenile Paget’s disease. N Engl J Med 2002, 347:175–184.
Cundy T, Hedge M, Naot D, et al.: A mutation in the gene TNFRSF11B encoding osteoprotegerin causes an idiopathic hyperphosphatasia phenotype. Hum Mol Genet 2002, 11:2119–2127.
Bolon B, Carter C, Daris M, et al.: Adenoviral delivery of osteoprotegerin ameliorates bone resorption in a mouse ovariectomy model of osteoporosis. Mol Ther 2001, 3:197–205.
Kostenuik PJ, Capparelli C, Morony S, et al.: OPG and PTH-(1-34) have additive effects on bone density and mechanical strength in osteopenic ovariectomized rats. Endocrinology 2001, 142:4295–4304.
Shimizu-Ishiura M, Kawana F, Sasaki T: Osteoprotegerin administration reduces femural bone loss in ovariectomized mice via impairment of osteoclast structure and function. J Electron Microsc 2002, 51:315–325.
Bateman TA, Dunstan CR, Ferguson VL, et al.: Osteoprotegerin mitigates tail suspension-induced osteopenia. Bone 2000, 26:443–449.
Kosternuik PJ, Ominsky MS, Cramner P, et al.: The RANKL antagonist OPG-Fc causes significant increases in cortical bone thickness, density and bone strength index in adult cynomolgus monkeys. Osteoporos Int 2005, 16(Suppl3):S68.
Black DM, Greenspan SL, Ensrud KE, et al.: The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med 2003, 349:1207–1215.
Morony S, Lu J, Capparelli C, et al.: Osteoprotegerin prevents bone loss in a rat model of glucocorticoid-induced osteopenia. J Bone Miner Res 2001, 16:S148.
Romas E, Sims NA, Hards DK, et al.: Osteoprotegerin reduces osteoclast numbers and prevents bone erosion in collageninduced arthritis. Am J Pathol 2002, 161:1419–1427.
Clohisy JC, Roy BC, Biondo C, et al.: Direct inhibition of NF-kappa B blocks bone erosion associated in inflammatory arthritis. J Immunol 2003, 171:5547–5553. This study and the article by Romas et al. [38] suggest that inhibition of RANKL may be effective in treating the bony erosions associated with inflammatory joint diseases, a clinical condition that has, to date, been refractory to other therapies.
Morony S, Capparelli C, Sarosi I, et al.: Osteoprotegerin inhibits osteolysis and decreases skeletal tumor burden in syngeneic and nude mouse models of experimental bone metastasis. Cancer Res 2001, 61:4432–4436.
Morony S, Capparelli C, Lee R, Shimamoto G, et al.: A chimeric form of osteoprotegerin inhibits hypercalcemia and bone resorption induced by IL-1β, TNF-α, PTH, PTHrP, and 1.25(OH)2D3. J Bone Miner Res 1999, 14:1478–1485.
Capparelli C, Kostenuik PJ, Morony S, et al.: Osteoprotegerin prevents and reverses hypercalcemia in a murine model of humoral hypercalcemia of malignancy. Cancer Res 2000, 60:783–778.
Morony S, Warmington K, Adamu S, et al.: The inhibition of RANKL causes greater suppression of bone resorption and hypercalcemia compared with bisphosphonates in two models of humoral hypercalcemia of malignancy. Endocrinology 2005, 146:3235–3243.
Bekker PJ, Holloway D, Nakanishi A, et al.: The effect of a single dose of osteoprotegerin in postmenopausal women. J Bone Miner Res 2001, 16:348–360. This important study documented the possibility of inhibiting RANKL-induced bone resorption in humans.
Body JJ, Greipp P, Coleman RE, et al.: A phase I study of AMGN-0007, a recombinant osteoprotegerin construct, in patients with multiple myeloma or breast carcinoma related bone metastases. Cancer 2003, 97:887–892. Significant inhibition of bone resorption occurred after OPG administration to patients with cancer-related bone diseases, lending promise to the usefulness of RANKL inhibitors in these patients.
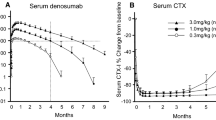
Bekker PJ, Holloway DL, Rasmussen AS, et al.: A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res 2004, 19:1059–1066. This phase I study documented that a specific anti-RANKL antibody resulted in rapid and sustained inhibition of bone turnover in humans, that this therapy was well tolerated and that long intervals between doses might be possible.
McClung MR, Lewiecki EM, Bolognese MA, et al.: AMG 162 increases bone mineral density (BMD) within 1 month in postmenopausal women with low BMD. J Bone Miner Res 2004, 19(Suppl1):S20. This is the preliminary report of the phase II study evaluating the effect of denosumab (AMG 162) on bone density.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McClung, M.R. Inhibition of RANKL as a treatment for osteoporosis: Preclinical and early clinical studies. Curr Osteoporos Rep 4, 28–33 (2006). https://doi.org/10.1007/s11914-006-0012-7
Issue Date:
DOI: https://doi.org/10.1007/s11914-006-0012-7