Abstract
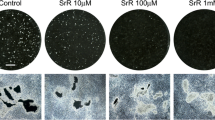
In vitro, strontium ranelate increases collagen and noncollagen protein synthesis by mature osteoblast-enriched cells. Its effects on bone formation were confirmed as the drug enhanced preosteoblastic cell replication. In the isolated osteoclast, preincubation of bone slices with strontium ranelate-induced dose-dependent inhibition of the bone-resorbing activity of treated rat osteoclast. Strontium ranelate dose-dependently inhibited preosteoclast differentiation. Its effect in postmenopausal women with established osteoporosis was assessed during an international, prospective, double-blind, randomized, placebo-controlled phase 3 program comparing strontium ranelate 2 g daily with placebo. The 3-year analysis of the phase 3 study, Spinal Osteoporosis Therapeutic Intervention, evaluating the effect of strontium ranelate 2 g/day on vertebral fracture rates, revealed a significant 41% reduction in the relative risk of patients experiencing new vertebral fracture with strontium ranelate over 3 years. A second phase 3 study showed a significant reduction in the relative risk of experiencing a nonvertebral fracture in the group treated with strontium ranelate over 3 years. These results show that strontium ranelate is a new, effective, and safe treatment for vertebral and hip osteoporosis, with a unique mode of action, increasing bone formation and decreasing bone resorption leading to a rebalance of bone turnover in favor of bone formation.
Similar content being viewed by others
References and Recommended Reading
Canalis E, Hott M, Deloffre P, et al.: The divalent strontium salt S12911 enhances bone cell replication and bone formation in vitro. Bone 1996, 18:517–523.
Marie PJ, Ammann P, Boivin G, Rey C: Mechanisms of action and therapeutic potential of strontium in bone. Calcif Tissue Int 2001, 69:121–129.
Izumisawa T, Morohashi T, Amano H, Yamada S: The effect of stable strontium on calcium metabolism: II. Effect of 1-hydroxyvitamin D3 in strontium-fed rats and inhibitory effect of strontium on bone resorption in vitro. J Bone Miner Metab 1994, 12:43–49.
Takahashi N, Sasaki T, Tsouderos Y, Suda T: S12911-2 inhibits osteoclastic bone resorption in vitro. J Bone Miner Res 2003, 18:1082–1087.
Baron R, Tsouderos Y: In vitro effects of S12911-2 on osteo-clast function and bone marrow macrophage differentiation. Eur J Pharmacol 2002, 450:11–17.
Buehler J, Chappuis P, Saffar JL, et al.: Strontium ranelate inhibits bone resorption while maintaining bone formation in alveolar bone in monkeys (Macaca fascicularis). Bone 2001, 29:176–179.
Boivin G, Deloffre P, Perrat B, et al.: Strontium distribution and interactions with bone mineral density in monkey iliac bone after strontium salt (S12911) administration. J Bone Miner Res 1996, 11:1302–1311.
Morohashi T, Sano T, Harai K, Yamada S: Effects of strontium on calcium metabolism in rats II. Strontium prevents the increased rate of bone turnover in ovariectomized rats. Jpn J Pharmacol 1995, 68:153–159.
Marie PJ, Hott M, Modrowski D, et al.: An uncoupling agent containing strontium prevents bone loss by depressing bone resorption and maintining bone formation in estrogen-deficient rats. J Bone Miner Res 1993, 8:607–615.
Hott M, Deloffre P, Tsouderos Y, Marie PJ: S12911-2 reduces bone loss induced by short-term immobilization in rats. Bone 2003, 33:115–123.
Meunier PJ, Slosman D, Delmas P, et al.: Strontium ranelate: dose-dependent effects in established postmenopausal vertebral osteoporosis: a 2-year randomized placebo controlled trial. J Clin Endocrinol Metab 2002, 87:2060–2066. The first trial showing an effect on strontium ranelate on BMD and biochemical markers of bone turnover in postmenopausal women.
Boivin G, Foos E, Tupinon-Mathieu I, Meunier PJ: Strontium deposition in bone is dose dependent and does not alter the degree of mineralization of bone in osteoporotic patients treated with strontium ranelate. J Bone Miner Res 2000, 15(Suppl 1):305.
Reginster JY, Diez-Perez A, Ortolani S, et al.: Calcium-vitamin D supplementation in clinical trials of osteoporosis should be titrated on the basis of pre-study assessments. Osteoporos Int 2002, 13(Suppl 1):S24.
Reginster JY, Spector T, Badurski J, et al.: A short-term run-in study can significantly contribute to increasing the quality of long-term osteoporosis trials. The strontium ranelate Phase III program. Osteoporos Int 2002, 13(Suppl 1):S30.
Genant HK, Wu CY, Van Kuijk C, Newit MC: Vertebral semi quantitative assessment using a semi-quantitative technique. J Bone Miner Res 1993, 8:1137–1148.
Meunier PJ, Roux Ortolani S, et al.: Strontium ranelate reduces the vertebral fracture risk in women with postmenopausal osteoporosis. Osteoporos Int 2002, 13(Suppl 1):O45.
Meunier PJ, Roux Seeman E, et al.: The effect of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med 2004, 350:459–468. A prospective double-blind randomized study demonstrating the antifracture efficacy of strontium ranelate, in spinal osteoporosis in postmenopausal women.
Rizzoli R, Reginster JY, Diaz-Curiel M, et al.: Patients at high risk of hip fracture benefit from treatment with strontium ranelate. Osteoporos Int 2004, 15(Suppl 1):OC39.
Reginster JY, Rizzoli R, Balogh A, et al.: Strontium ranelate reduces the risk of vertebral fractures in osteoporotic post menopausal women without prevalent vertebral fracture. Calcif Tissue Int 2004, 74(Suppl 1):P151.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reginster, JY., Sarlet, N., Lejeune, E. et al. Strontium ranelate: A new treatment for postmenopausal osteoporosis with a dual mode of action. Curr Osteoporos Rep 3, 30–34 (2005). https://doi.org/10.1007/s11914-005-0025-7
Issue Date:
DOI: https://doi.org/10.1007/s11914-005-0025-7