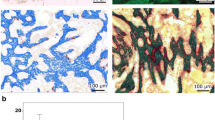
Abstract
Strontium ranelate (SrR) has both bone anabolic and anti-resorption properties and has therefore the potential to increase the healing of bone defects. The aim of the present study was to investigate the effect of systemic treatment with SrR during the healing of cortical bone defects in rats. In addition, the vertebral bodies were examined in order to elucidate the effect of short-term treatment with SrR on intact trabecular bone. Sixty 16-week-old female Wistar rats were randomized into four groups. A cylindrical defect was drilled through the anterior cortex of the mid-femoral diaphysis in both hind limbs. Two of the groups were treated with SrR (900 mg/kg b.w.) mixed into the food and two groups served as controls. The animals were euthanized after either 3 or 8 weeks of treatment. Healing of the defects was analyzed with µCT, mechanical testing, and stereology. Treatment with SrR resulted in increased thickness of the defects after 3 weeks of treatment, whereas no effect on bone volume fraction (BV/TV), mechanical properties (maximum strength and maximum stiffness), periosteal callus volume, or osteoclast-covered bone surfaces (Oc.S/BS) after either 3 or 8 weeks of treatment was found. Furthermore, SrR increased the bone material density (ρ) of the vertebral bodies, and tended to increase BV/TV after 8 weeks of treatment (p = 0.087). The mechanical properties of the vertebral bodies were not influenced by SrR treatment. In conclusion, 3 weeks of treatment with SrR increased the thickness of the healing mid-femoral cortical bone defects in rats, but did not influence BV/TV, mechanical properties, periosteal callus volume, or Oc.S/BS after either 3 or 8 weeks. Furthermore, SrR had no effect on the microstructure and mechanical properties of the vertebral bodies.



Similar content being viewed by others
References
Pipitone PS, Rehman S (2014) Management of traumatic bone loss in the lower extremity. Orthop Clin North Am 45:469–482. doi:10.1016/j.ocl.2014.06.008
Gaston MS, Simpson AH (2007) Inhibition of fracture healing. J Bone Joint Surg Br 89:1553–1560. doi:10.1302/0301-620X.89B12.19671
Wiese A, Pape HC (2010) Bone defects caused by high-energy injuries, bone loss, infected nonunions, and nonunions. Orthop Clin North Am 41:1–4. doi:10.1016/j.ocl.2009.07.003
Zeckey C, Mommsen P, Andruszkow H et al (2011) The aseptic femoral and tibial shaft non-union in healthy patients—an analysis of the health-related quality of life and the socioeconomic outcome. Open Orthop J 5:193–197. doi:10.2174/1874325001105010193
Jørgensen NR, Schwarz P (2011) Effects of anti-osteoporosis medications on fracture healing. Curr Osteoporos Rep 9:149–155. doi:10.1007/s11914-011-0065-0
Larsson S, Fazzalari NL (2014) Anti-osteoporosis therapy and fracture healing. Arch Orthop Trauma Surg 134:291–297. doi:10.1007/s00402-012-1558-8
Gerstenfeld LC, Sacks DJ, Pelis M et al (2009) Comparison of effects of the bisphosphonate alendronate versus the RANKL inhibitor denosumab on murine fracture healing. J Bone Miner Res 24:196–208. doi:10.1359/jbmr.081113
Peichl P, Holzer LA, Maier R, Holzer G (2011) Parathyroid hormone 1-84 accelerates fracture-healing in pubic bones of elderly osteoporotic women. J Bone Joint Surg Am 93:1583–1587. doi:10.2106/JBJS.J.01379
Manabe T, Mori S, Mashiba T et al (2007) Human parathyroid hormone (1-34) accelerates natural fracture healing process in the femoral osteotomy model of cynomolgus monkeys. Bone 40:1475–1482. doi:10.1016/j.bone.2007.01.015
Campbell EJ, Campbell GM, Hanley DA (2015) The effect of parathyroid hormone and teriparatide on fracture healing. Expert Opin Biol Ther 15:119–129. doi:10.1517/14712598.2015.977249
Aspenberg P, Genant HK, Johansson T et al (2010) Teriparatide for acceleration of fracture repair in humans: a prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J Bone Miner Res 25:404–414. doi:10.1359/jbmr.090731
Marie PJ (2006) Strontium ranelate: a physiological approach for optimizing bone formation and resorption. Bone 38:S10–S14. doi:10.1016/j.bone.2005.07.029
Bonnelye E, Chabadel A, Saltel F, Jurdic P (2008) Dual effect of strontium ranelate: stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone 42:129–138. doi:10.1016/j.bone.2007.08.043
Stepan JJ (2013) Strontium ranelate: in search for the mechanism of action. J Bone Miner Metab 31:606–612. doi:10.1007/s00774-013-0494-1
Vegger JB, Nielsen ES, Brüel A, Thomsen JS (2014) Additive effect of PTH (1-34) and zoledronate in the prevention of disuse osteopenia in rats. Bone 66:287–295. doi:10.1016/j.bone.2014.06.020
Brüel A, Olsen J, Birkedal H et al (2010) Strontium is incorporated into the fracture callus but does not influence the mechanical strength of healing rat fractures. Calcif Tissue Int 88:142–152. doi:10.1007/s00223-010-9439-z
Habermann B, Kafchitsas K, Olender G et al (2009) Strontium ranelate enhances callus strength more than PTH 1-34 in an osteoporotic rat model of fracture healing. Calcif Tissue Int 86:82–89. doi:10.1007/s00223-009-9317-8
Brüel A, Vegger JB, Raffalt AC et al (2013) PTH (1-34), but not strontium ranelate counteract loss of trabecular thickness and bone strength in disuse osteopenic rats. Bone 53:51–58. doi:10.1016/j.bone.2012.11.037
Sikjaer T, Rejnmark L, Thomsen JS et al (2012) Changes in 3-dimensional bone structure indices in hypoparathyroid patients treated with PTH(1-84): a randomized controlled study. J Bone Miner Res 27:781–788. doi:10.1002/jbmr.1493
Bouxsein ML, Boyd SK, Christiansen BA et al (2010) Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25:1468–1486. doi:10.1002/jbmr.141
Thomsen JS, Laib A, Koller B et al (2005) Stereological measures of trabecular bone structure: comparison of 3D micro computed tomography with 2D histological sections in human proximal tibial bone biopsies. J Microsc 218:171–179. doi:10.1111/j.1365-2818.2005.01469.x
Gundersen HJ, Jensen EB (1987) The efficiency of systematic sampling in stereology and its prediction. J Microsc 147:229–263
Cebesoy O, Tutar E, Kose KC et al (2007) Effect of strontium ranelate on fracture healing in rat tibia. Jt Bone Spine 74:590–593. doi:10.1016/j.jbspin.2007.01.034
Li YF, Luo E, Feng G et al (2010) Systemic treatment with strontium ranelate promotes tibial fracture healing in ovariectomized rats. Osteoporos Int 21:1889–1897. doi:10.1007/s00198-009-1140-6
Ozturan KE, Demir B, Yucel I et al (2011) Effect of strontium ranelate on fracture healing in the osteoporotic rats. J Orthop Res 29:138–142. doi:10.1002/jor.21204
Komrakova M, Weidemann A, Dullin C et al (2015) The impact of strontium ranelate on metaphyseal bone healing in ovariectomized Rats. Calcif Tissue Int. doi:10.1007/s00223-015-0019-0
Lin K, Xia L, Li H et al (2013) Enhanced osteoporotic bone regeneration by strontium-substituted calcium silicate bioactive ceramics. Biomaterials 34:10028–10042. doi:10.1016/j.biomaterials.2013.09.056
Thormann U, Ray S, Sommer U et al (2013) Bone formation induced by strontium modified calcium phosphate cement in critical-size metaphyseal fracture defects in ovariectomized rats. Biomaterials 34:8589–8598. doi:10.1016/j.biomaterials.2013.07.036
Wei L, Ke J, Prasadam I et al (2014) A comparative study of Sr-incorporated mesoporous bioactive glass scaffolds for regeneration of osteopenic bone defects. Osteoporos Int 25:2089–2096. doi:10.1007/s00198-014-2735-0
Zacchetti G, Dayer R, Rizzoli R, Ammann P (2014) Systemic treatment with strontium ranelate accelerates the filling of a bone defect and improves the material level properties of the healing bone. Biomed Res Int 2014:549785. doi:10.1155/2014/549785
Komatsu DE, Brune KA, Liu H et al (2009) Longitudinal in vivo analysis of the region-specific efficacy of parathyroid hormone in a rat cortical defect model. Endocrinology 150:1570–1579. doi:10.1210/en.2008-0814
Einhorn TA (1998) The cell and molecular biology of fracture healing. Clin Orthop Relat Res (355 Suppl):S7–21
Monfoulet L, Rabier B, Chassande O, Fricain JC (2010) Drilled hole defects in mouse femur as models of intramembranous cortical and cancellous bone regeneration. Calcif Tissue Int 86:72–81. doi:10.1007/s00223-009-9314-y
Ammann P, Shen V, Robin B et al (2004) Strontium ranelate improves bone resistance by increasing bone mass and improving architecture in intact female rats. J Bone Miner Res 19:2012–2020. doi:10.1359/JBMR.040906
Boyd SK, Szabo E, Ammann P (2011) Increased bone strength is associated with improved bone microarchitecture in intact female rats treated with strontium ranelate: A finite element analysis study. Bone 48:1109–1116. doi:10.1016/j.bone.2011.01.004
Grynpas MD, Hamilton E, Cheung R et al (1996) Strontium increases vertebral bone volume in rats at a low dose that does not induce detectable mineralization defect. Bone 18:253–259. doi:10.1016/8756-3282(95)00484-X
Sun P, Cai DH, Li QN et al (2010) Effects of alendronate and strontium ranelate on cancellous and cortical bone mass in glucocorticoid-treated adult rats. Calcif Tissue Int 86:495–501. doi:10.1007/s00223-010-9363-2
Wu Y, Adeeb SM, John Duke M et al (2013) Compositional and material properties of rat bone after bisphosphonate and/or strontium ranelate drug treatment. J Pharm Pharm Sci 16:52–64
Acknowledgments
The authors are grateful for the hard work and excellent technical assistance of Jytte Utoft. Thomas Dalager Jørgensen is acknowledged for his elegant graphical work. We thank Visiopharm for the contribution to the newCAST stereology software system and the Velux Foundation for the donation of the µCT scanner. The study was kindly supported by the Aarhus University Research Foundation and the Danish Council for Independent Research | Medical Sciences (0602-01706B).
Contributor Statement
JBV, AB, and JST designed the study. JBV, AB, TGS, and JST contributed to the experimental work. JBV prepared the first draft of the paper and is the guarantor. All authors revised the paper critically for intellectual content and approved the final version. All authors agree to be accountable for the work and to ensure that any questions relating to the accuracy and integrity of the paper are investigated and properly resolved.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors Jens Bay Vegger, Annemarie Brüel, Thomas Givskov Sørensen, and Jesper Skovhus Thomsen declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
The experiment complied with the EU Directive 2010/63/EU for animal experiments, and all procedures were approved by the Danish Animal Inspectorate under the Danish Ministry of Justice.
Electronic supplementary material
Below is the link to the electronic supplementary material.
223_2015_77_MOESM1_ESM.pdf
Information about chow intake, Strontium Ranelate concentration in the chow, and Strontium Ranelate intake by the rats receiving Strontium Ranelate. Supplementary material 1 (PDF 84 kb)
Rights and permissions
About this article
Cite this article
Vegger, J.B., Brüel, A., Sørensen, T.G. et al. Systemic Treatment with Strontium Ranelate Does Not Influence the Healing of Femoral Mid-shaft Defects in Rats. Calcif Tissue Int 98, 206–214 (2016). https://doi.org/10.1007/s00223-015-0077-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-015-0077-3