Abstract
Purpose for Review
This perspective piece has two goals: first, to describe issues related to artificial intelligence-based applications for cancer control as they may impact health inequities or disparities; and second, to report on a review of systematic reviews and meta-analyses of artificial intelligence-based tools for cancer control to ascertain the extent to which discussions of justice, equity, diversity, inclusion, or health disparities manifest in syntheses of the field’s best evidence.
Recent Findings
We found that, while a significant proportion of existing syntheses of research on AI-based tools in cancer control use formal bias assessment tools, the fairness or equitability of models is not yet systematically analyzable across studies. Issues related to real-world use of AI-based tools for cancer control, such as workflow considerations, measures of usability and acceptance, or tool architecture, are more visible in the literature, but still addressed only in a minority of reviews.
Summary
Artificial intelligence is poised to bring significant benefits to a wide range of applications in cancer control, but more thorough and standardized evaluations and reporting of model fairness are required to build the evidence base for AI-based tool design for cancer and to ensure that these emerging technologies promote equitable healthcare.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bhalla S, Laganà A. Artificial intelligence for precision oncology. Adv Exp Med Biol. 2022;1361:249–68.
Fitzgerald J, Higgins D, Mazo Vargas C, et al. Future of biomarker evaluation in the realm of artificial intelligence algorithms: application in improved therapeutic stratification of patients with breast and prostate cancer. J Clin Pathol. 2021;74(7):429–34.
Shimizu H, Nakayama KI. Artificial intelligence in oncology. Cancer Sci. 2020;111(5):1452–60.
Matheny ME, Whicher D, Thadaney IS. Artificial intelligence in health care: a report from the national academy of medicine. JAMA. 2020;323(6):509–10. Extensive report on current state of artificial intelligence in medicine and needs for future research.
Chua IS, Gaziel-Yablowitz M, Korach ZT, et al. Artificial intelligence in oncology: Path to implementation. Cancer Med. 2021;10(12):4138–49.
Islami F, Guerra CE, Minihan A, et al. American Cancer Society’s report on the status of cancer disparities in the United States, 2021. CA Cancer J Clin. 2022;72(2):112–43. Important summary on the state of knowledge in cancer disparities.
Woo B, Kravitz-Wirtz N, Sass V, Crowder K, Teixeira S, Takeuchi DT. Residential segregation and racial/ethnic disparities in ambient air pollution. Race Soc Probl. 2019;11(1):60–7.
Agénor M, Pérez AE, Peitzmeier SM, Borrero S. Racial/ethnic disparities in human papillomavirus vaccination initiation and completion among U.S. women in the post-Affordable Care Act era. Ethn Health. 2020;25(3):393–407.
Yabroff KR, Dowling EC, Guy GP Jr, et al. Financial hardship associated with cancer in the United States: findings from a population-based sample of adult cancer survivors. J Clin Oncol. 2016;34(3):259–67.
Erdrich J, Cordova-Marks F, Monetathchi AR, Wu M, White A, Melkonian S. Disparities in breast-conserving therapy for non-Hispanic American Indian/Alaska Native Women Compared with Non-Hispanic White Women. Ann Surg Oncol. 2022;29(2):1019–30.
Krahn GL, Walker DK, Correa-De-Araujo R. Persons with disabilities as an unrecognized health disparity population. Am J Public Health. 2015;105(Suppl 2):S198-206.
Samtani G, Bassford TL, Williamson HJ, Armin JS. Are researchers addressing cancer treatment and survivorship among people with intellectual and developmental disabilities in the US? A Scoping Review. Intellect Dev Disabil. 2021;59(2):141–54.
Panch T, Mattie H, Atun R. Artificial intelligence and algorithmic bias: implications for health systems. J Glob Health. 2019;9(2): 010318.
Clark CR, Wilkins CH, Rodriguez JA, et al. Health care equity in the use of advanced analytics and artificial intelligence technologies in primary care. J Gen Intern Med. 2021;36(10):3188–93.
15. Selbst AD, Boyd D, Friedler SA, Venkatasubramanian S, Vertesi J. Fairness and abstraction in sociotechnical systems. In: Proceedings of the conference on fairness, accountability, and transparency. New York, NY :Association for Computing Machinery; 2019. p. 59–68.
Friedler SA, Scheidegger C, Venkatasubramanian S. The (im) possibility of fairness: Different value systems require different mechanisms for fair decision making. Commun ACM 2021;64.4:136–143.
DeCamp M, Lindvall C. Latent bias and the implementation of artificial intelligence in medicine. J Am Med Inform Assoc. 2020;27(12):2020–3.
Plascak JJ, Beyer K, Xu X, Stroup AM, Jacob G, Llanos AAM. Association between residence in historically redlined districts indicative of structural racism and racial and ethnic disparities in breast cancer outcomes. JAMA Netw Open. 2022;5(7): e2220908.
Sistrunk C, Tolbert N, Sanchez-Pino MD, et al. Impact of federal, state, and local housing policies on disparities in cardiovascular disease in Black/African American Men and Women: From Policy to Pathways to Biology. Front Cardiovasc Med. 2022;9: 756734.
Assari S, Bazargan M. Unequal effects of educational attainment on workplace exposure to second-hand smoke by race and ethnicity; Minorities' diminished returns in the National Health Interview Survey (NHIS). J Med Res Innov 2019;3(2):e000179. https://doi.org/10.32892/jmri.179.
Juon HS, Hong A, Pimpinelli M, Rojulpote M, McIntire R, Barta JA. Racial disparities in occupational risks and lung cancer incidence: analysis of the National Lung Screening Trial. Prev Med. 2021;143: 106355.
Ricks TN, Abbyad C, Polinard E. Undoing racism and mitigating bias among healthcare professionals: lessons learned during a systematic review. J Racial Ethn Health Disparities. 2022;9(5):1990–2000.
Schatz AA, Brooks-Coley K, Harrington E, Murray MS, Carlson RW. Patient, caregiver, and oncologist experiences with and perceptions of racial bias and discrimination in cancer care delivery. J Natl Compr Canc Netw. 2022;20(10):1092-1098.e1092.
Dankwa-Mullan I, Scheufele EL, Matheny ME, et al. A proposed framework on integrating health equity and racial justice into the artificial intelligence development lifecycle. J Health Care Poor Underserved. 2021;32(2):300–17.
Suresh H, Guttag J. A framework for understanding sources of harm throughout the machine learning life cycle. In: Equity and Access in Algorithms, Mechanisms, and Optimization (EAAMO '21). New York, NY: Association for Computing Machinery; 2021. p. 1–9. https://doi.org/10.1145/3465416.3483305.
Ng MY, Kapur S, Blizinsky KD, Hernandez-Boussard T. The AI life cycle: a holistic approach to creating ethical AI for health decisions. Nat Med. 2022;28(11):2247–9. https://doi.org/10.1038/s41591-022-01993-y.
Rogers WA, Draper H, Carter SM. Evaluation of artificial intelligence clinical applications: Detailed case analyses show value of healthcare ethics approach in identifying patient care issues. Bioethics. 2021;35(7):623–33.
Ibrahim H, Liu X, Zariffa N, Morris AD, Denniston AK. Health data poverty: an assailable barrier to equitable digital health care. Lancet Digit Health. 2021;3(4):e260–5.
Chavez-Yenter D, Goodman MS, Chen Y, et al. Association of disparities in family history and family cancer history in the electronic health record with sex, race, hispanic or latino ethnicity, and language preference in 2 large US Health Care Systems. JAMA Netw Open. 2022;5(10): e2234574.
Stern MC, Zhang J, Lee E, Deapen D, Liu L. Disparities in colorectal cancer incidence among Latino subpopulations in California defined by country of origin. Cancer Causes Control. 2016;27(2):147–55.
Cerni J, Rhee J, Hosseinzadeh H. End-of-life cancer care resource utilisation in rural versus urban settings: a systematic review. Int J Environ Res Public Health. 2020;17(14):4955.
Morris BB, Rossi B, Fuemmeler B. The role of digital health technology in rural cancer care delivery: A systematic review. J Rural Health. 2022;38(3):493–511.
Robertson NM, Hudson L, Attia SL, Porterfield JZ, Vanderford NL. Assessing the effectiveness of cancer screening interventions targeting appalachian populations: a systematic review. J Rural Health. 2021;37(3):602–23.
Palmer Kelly E, McGee J, Obeng-Gyasi S, et al. Marginalized patient identities and the patient-physician relationship in the cancer care context: a systematic scoping review. Support Care Cancer. 2021;29(12):7195–207.
Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019;366(6464):447–53. Important case study in racial bias in artificial intelligence-based decision support.
Obermeyer Z, Topol EJ. Artificial intelligence, bias, and patients’ perspectives. Lancet. 2021;397(10289):2038.
Bogner J, Verdecchia R, Gerostathopoulos I. Characterizing technical debt and antipatterns in AI-based systems: a systematic mapping study. In: 2021 IEEE/ACM International Conference on Technical Debt. Madrid, Spain: TechDebt; 2021. p. 64-73. https://doi.org/10.1109/TechDebt52882.2021.00016.
Finlayson SG, Subbaswamy A, Singh K, et al. The clinician and dataset shift in artificial intelligence. N Engl J Med. 2021;385(3):283–6.
London AJ. Artificial intelligence in medicine: Overcoming or recapitulating structural challenges to improving patient care? Cell Rep Med. 2022;3(5): 100622.
Lipton ZC. The mythos of model interpretability: In machine learning, the concept of interpretability is both important and slippery. Queue. 2018;16(3):31–57.
Burrell J. How the machine ‘thinks’: understanding opacity in machine learning algorithms. Big Data Soc. 2016;3(1):2053951715622512.
Hauser K, Kurz A, Haggenmüller S, et al. Explainable artificial intelligence in skin cancer recognition: A systematic review. Eur J Cancer. 2022;167:54–69.
Loh HW, Ooi CP, Seoni S, Barua PD, Molinari F, Acharya UR. Application of explainable artificial intelligence for healthcare: a systematic review of the last decade (2011–2022). Comput Methods Programs Biomed. 2022;226: 107161.
Payrovnaziri SN, Chen Z, Rengifo-Moreno P, et al. Explainable artificial intelligence models using real-world electronic health record data: a systematic scoping review. J Am Med Inform Assoc. 2020;27(7):1173–85.
Vo TH, Nguyen NTK, Kha QH, Le NQK. On the road to explainable AI in drug-drug interactions prediction: A systematic review. Comput Struct Biotechnol J. 2022;20:2112–23.
Wells L, Bednarz T. Explainable AI and reinforcement learning-a systematic review of current approaches and trends. Front Artif Intell. 2021;4: 550030.
TulkJesso S, Kelliher A, Sanghavi H, Martin T, Henrickson PS. Inclusion of clinicians in the development and evaluation of clinical artificial intelligence tools: a systematic literature review. Front Psychol. 2022;13: 830345.
Seneviratne MG, Li RC, Schreier M, et al. User-centred design for machine learning in health care: a case study from care management. BMJ Health Care Inform. 2022;29(1):e100656.
Wang L, Chignell M, Zhang Y, et al. Physician experience design (PXD): more usable machine learning prediction for clinical decision making. AMIA Annu Symp Proc. 2022;2022:476–85.
Murad MH, Asi N, Alsawas M, Alahdab F. New evidence pyramid. Evid Based Med. 2016;21(4):125–7.
Bedrikovetski S, Dudi-Venkata NN, Kroon HM, et al. Artificial intelligence for pre-operative lymph node staging in colorectal cancer: a systematic review and meta-analysis. BMC Cancer. 2021;21(1):1058.
Hassan C, Spadaccini M, Iannone A, et al. Performance of artificial intelligence in colonoscopy for adenoma and polyp detection: a systematic review and meta-analysis. Gastrointest Endosc. 2021;93(1):77-85.e76.
Nazarian S, Glover B, Ashrafian H, Darzi A, Teare J. Diagnostic accuracy of artificial intelligence and computer-aided diagnosis for the detection and characterization of colorectal polyps: systematic review and meta-analysis. J Med Internet Res. 2021;23(7): e27370.
Staal FCR, van der Reijd DJ, Taghavi M, Lambregts DMJ, Beets-Tan RGH, Maas M. Radiomics for the prediction of treatment outcome and survival in patients with colorectal cancer: a systematic review. Clin Colorectal Cancer. 2021;20(1):52–71.
Xu Y, Ding W, Wang Y, et al. Comparison of diagnostic performance between convolutional neural networks and human endoscopists for diagnosis of colorectal polyp: a systematic review and meta-analysis. PLoS ONE. 2021;16(2): e0246892.
Chuchu N, Takwoingi Y, Dinnes J, et al. Smartphone applications for triaging adults with skin lesions that are suspicious for melanoma. Cochrane Database Syst Rev. 2018;12(12):Cd013192.
Deliwala SS, Hamid K, Barbarawi M, et al. Artificial intelligence (AI) real-time detection vs. routine colonoscopy for colorectal neoplasia: a meta-analysis and trial sequential analysis. Int J Colorectal Dis. 2021;36(11):2291–303.
di Ruffano LF, Takwoingi Y, Dinnes J, et al. Computer-assisted diagnosis techniques (dermoscopy and spectroscopy-based) for diagnosing skin cancer in adults. Cochrane Database Syst Rev. 2018;12(12):Cd013186.
Jones OT, Matin RN, van der Schaar M, et al. Artificial intelligence and machine learning algorithms for early detection of skin cancer in community and primary care settings: a systematic review. Lancet Digit Health. 2022;4(6):e466–76. Systematic review of artificial intelligence-based decision support for skin cancer detection with clear discussion of risks for unfair model performance.
Marka A, Carter JB, Toto E, Hassanpour S. Automated detection of nonmelanoma skin cancer using digital images: a systematic review. BMC Med Imaging. 2019;19(1):21.
Jones OT, Calanzani N, Saji S, et al. Artificial intelligence techniques that may be applied to primary care data to facilitate earlier diagnosis of cancer: systematic review. J Med Internet Res. 2021;23(3): e23483.
Klarenbeek SE, Weekenstroo HHA, Sedelaar JPM, Fütterer JJ, Prokop M, Tummers M. The Effect of higher level computerized clinical decision support systems on oncology care: a systematic review. Cancers (Basel). 2020;12(4):1032.
Lu SC, Xu C, Nguyen CH, Geng Y, Pfob A, Sidey-Gibbons C. Machine learning-based short-term mortality prediction models for patients with cancer using electronic health record data: systematic review and critical appraisal. JMIR Med Inform. 2022;10(3): e33182.
Popescu ER, Geantă M, Brand A. Mapping of clinical research on artificial intelligence in the treatment of cancer and the challenges and opportunities underpinning its integration in the European Union health sector. Eur J Public Health. 2022;32(3):443–9.
Rezayi S, R Niakan Kalhori S, Saeedi S. Effectiveness of artificial intelligence for personalized medicine in neoplasms: a systematic review. Biomed Res Int. 2022;2022:7842566. https://doi.org/10.1155/2022/7842566.
Xu L, Sanders L, Li K, Chow JCL. Chatbot for health care and oncology applications using artificial intelligence and machine learning: systematic review. JMIR Cancer. 2021;7(4): e27850.
Yung A, Kay J, Beale P, Gibson KA, Shaw T. Computer-based decision tools for shared therapeutic decision-making in oncology: systematic review. JMIR Cancer. 2021;7(4): e31616.
Chidambaram S, Sounderajah V, Maynard N, Markar SR. Diagnostic performance of artificial intelligence-centred systems in the diagnosis and postoperative surveillance of upper gastrointestinal malignancies using computed tomography imaging: a systematic review and meta-analysis of diagnostic accuracy. Ann Surg Oncol. 2022;29(3):1977–90.
Corti C, Cobanaj M, Marian F, et al. Artificial intelligence for prediction of treatment outcomes in breast cancer: Systematic review of design, reporting standards, and bias. Cancer Treat Rev. 2022;108: 102410.
Dumitrescu EA, Ungureanu BS, Cazacu IM, et al. Diagnostic value of artificial intelligence-assisted endoscopic ultrasound for pancreatic cancer: a systematic review and meta-analysis. Diagnostics (Basel). 2022;12(2):309.
Huang G, Wei X, Tang H, Bai F, Lin X, Xue D. A systematic review and meta-analysis of diagnostic performance and physicians’ perceptions of artificial intelligence (AI)-assisted CT diagnostic technology for the classification of pulmonary nodules. J Thorac Dis. 2021;13(8):4797–811.
Syer T, Mehta P, Antonelli M, et al. Artificial intelligence compared to radiologists for the initial diagnosis of prostate cancer on magnetic resonance imaging: a systematic review and recommendations for future studies. Cancers (Basel). 2021;13(13):3318.
Hickman SE, Woitek R, Le EPV, et al. Machine learning for workflow applications in screening mammography: systematic review and meta-analysis. Radiology. 2022;302(1):88–104. Meta-analysis of artificial intelligence-based decision support for screening mammography with expolicit discussion of existing screening workflows.
Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36.
Wolff RF, Moons KGM, Riley RD, et al. PROBAST: A tool to assess the risk of bias and applicability of prediction model studies. Ann Intern Med. 2019;170(1):51–8.
Bates DW, Auerbach A, Schulam P, Wright A, Saria S. Reporting and implementing interventions involving machine learning and artificial intelligence. Ann Intern Med. 2020;172(11 Suppl):S137-s144. Accessible explanation of current artificial intelligence-based approaches in clinical decision support, and their related potential pitfalls and biases.
Collins GS, Dhiman P, Andaur Navarro CL, et al. Protocol for development of a reporting guideline (TRIPOD-AI) and risk of bias tool (PROBAST-AI) for diagnostic and prognostic prediction model studies based on artificial intelligence. BMJ Open. 2021;11(7): e048008.
Röösli E, Bozkurt S, Hernandez-Boussard T. Peeking into a black box, the fairness and generalizability of a MIMIC-III benchmarking model. Sci Data. 2022;9(1):24. An evaluation finding various forms of unfair performance in an open access benchmarking model, and calling for greater thoroughness and transparency in reporting of artificial intelligence-based tools.
Hernandez-Boussard T, Bozkurt S, Ioannidis JPA, Shah NH. MINIMAR (MINimum Information for Medical AI Reporting): Developing reporting standards for artificial intelligence in health care. J Am Med Inform Assoc. 2020;27(12):2011–5. A reporting format intended to provide sufficient granularity to evaluate fairness of model performance for subpopulations.
Goon P, Banfield C, Bello O, Levell NJ. Skin cancers in skin types IV-VI: Does the Fitzpatrick scale give a false sense of security? Skin Health Dis. 2021;1(3): e40.
Jamali H, Castillo LT, Morgan CC, et al. Racial disparity in oxygen saturation measurements by pulse oximetry: evidence and implications. Ann Am Thorac Soc. 2022;19(12):1951–64.
Valbuena VSM, Seelye S, Sjoding MW, et al. Racial bias and reproducibility in pulse oximetry among medical and surgical inpatients in general care in the Veterans Health Administration 2013–19: multicenter, retrospective cohort study. BMJ. 2022;378: e069775.
Li RC, Asch SM, Shah NH. Developing a delivery science for artificial intelligence in healthcare. NPJ Digit Med. 2020;3:107.
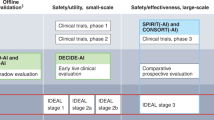
Vasey BCD, Collins GS, Denniston AK, Faes L, Geerts BF, Liu X, Morgan L, Watkinson P, McCulloch P, DECIDE-AI Steering Group. DECIDE-AI: new reporting guidelines to bridge the development-to-implementation gap in clinical artificial intelligence. Nat Med. 2021;27(2):186–7.
Sittig DF, Singh H. A new sociotechnical model for studying health information technology in complex adaptive healthcare systems. Qual Saf Health Care. 2010;19(Suppl 3):i68-74.
Fihn S, Saria S, Mendonça E, Hain E, Matheny M, Shah N, Liu H, Auerbach A. Deploying AI in clinical settings. In: Matheny M et al., editors. Artificial Intelligence in Health Care: The Hope, the Hype, the Promise, the Peril. National Academy of Medicine; 2019.
Topol EJ. Welcoming new guidelines for AI clinical research. Nat Med. 2020;26(9):1318–20.
Shelmerdine SC, Arthurs OJ, Denniston A, Sebire NJ. Review of study reporting guidelines for clinical studies using artificial intelligence in healthcare. BMJ Health Care Inform. 2021;28(1):e100385. https://doi.org/10.1136/bmjhci-2021-100385.
de Hond AAH, van Buchem MM, Hernandez-Boussard T. Picture a data scientist: a call to action for increasing diversity, equity, and inclusion in the age of AI. J Am Med Inform Assoc. 2022;29(12):2178–81. https://doi.org/10.1093/jamia/ocac156.
Nundy S, Cooper LA, Mate KS. The quintuple aim for health care improvement: a new imperative to advance health equity. JAMA. 2022;327(6):521–2.
Sittig DF, Lakhani P, Singh H. Applying requisite imagination to safeguard electronic health record transitions. J Am Med Inform Assoc. 2022;29(5):1014–8.
Boyarskaya M, Olteanu A, Crawford K. Overcoming failures of imagination in AI infused system development and deployment. ArXiv 2020;abs/2011.13416. https://doi.org/10.48550/arXiv.2011.13416
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Palliative Medicine
Appendix
Appendix
All Included Reviewed Systematic Reviews and Meta-analyses
-
1.
Adeoye J, Tan JY, Choi SW, Thomson P. Prediction models applying machine learning to oral cavity cancer outcomes: A systematic review. Int J Med Inform. 2021;154:104557.
-
2.
Akazawa M, Hashimoto K. Artificial intelligence in gynecologic cancers: Current status and future challenges - A systematic review. Artif Intell Med. 2021;120:102164.
-
3.
Alabi RO, Bello IO, Youssef O, Elmusrati M, Mäkitie AA, Almangush A. Utilizing Deep Machine Learning for Prognostication of Oral Squamous Cell Carcinoma-A Systematic Review. Front Oral Health. 2021;2:686863.
-
4.
Alabi RO, Youssef O, Pirinen M, et al. Machine learning in oral squamous cell carcinoma: Current status, clinical concerns and prospects for future-A systematic review. Artif Intell Med. 2021;115:102060.
-
5.
Anderson AW, Marinovich ML, Houssami N, et al. Independent External Validation of Artificial Intelligence Algorithms for Automated Interpretation of Screening Mammography: A Systematic Review. J Am Coll Radiol. 2022;19(2 Pt A):259-273.
-
6.
Aziz M, Fatima R, Dong C, Lee-Smith W, Nawras A. The impact of deep convolutional neural network-based artificial intelligence on colonoscopy outcomes: A systematic review with meta-analysis. J Gastroenterol Hepatol. 2020;35(10):1676-1683.
-
7.
Bang CS, Lee JJ, Baik GH. Computer-aided diagnosis of esophageal cancer and neoplasms in endoscopic images: a systematic review and meta-analysis of diagnostic test accuracy. Gastrointest Endosc. 2021;93(5):1006-1015.e1013.
-
8.
Barua I, Vinsard DG, Jodal HC, et al. Artificial intelligence for polyp detection during colonoscopy: a systematic review and meta-analysis. Endoscopy. 2021;53(3):277-284.
-
9.
Bedrikovetski S, Dudi-Venkata NN, Kroon HM, et al. Artificial intelligence for pre-operative lymph node staging in colorectal cancer: a systematic review and meta-analysis. BMC Cancer. 2021;21(1):1058.
-
10.
Bhandari A, Marwah R, Smith J, et al. Machine learning imaging applications in the differentiation of true tumour progression from treatment-related effects in brain tumours: A systematic review and meta-analysis. J Med Imaging Radiat Oncol. 2022;66(6):781-797.
-
11.
Chen PC, Lu YR, Kang YN, Chang CC. The Accuracy of Artificial Intelligence in the Endoscopic Diagnosis of Early Gastric Cancer: Pooled Analysis Study. J Med Internet Res. 2022;24(5):e27694.
-
12.
Chidambaram S, Sounderajah V, Maynard N, Markar SR. Diagnostic Performance of Artificial Intelligence-Centred Systems in the Diagnosis and Postoperative Surveillance of Upper Gastrointestinal Malignancies Using Computed Tomography Imaging: A Systematic Review and Meta-Analysis of Diagnostic Accuracy. Ann Surg Oncol. 2022;29(3):1977-1990.
-
13.
Chiesa-Estomba CM, Graña M, Medela A, et al. Machine Learning Algorithms as a Computer-Assisted Decision Tool for Oral Cancer Prognosis and Management Decisions: A Systematic Review. ORL J Otorhinolaryngol Relat Spec. 2022;84(4):278-288.
-
14.
Cho SJ, Sunwoo L, Baik SH, Bae YJ, Choi BS, Kim JH. Brain metastasis detection using machine learning: a systematic review and meta-analysis. Neuro Oncol. 2021;23(2):214-225.
-
15.
Chuchu N, Takwoingi Y, Dinnes J, et al. Smartphone applications for triaging adults with skin lesions that are suspicious for melanoma. Cochrane Database Syst Rev. 2018;12(12):Cd013192.
-
16.
Corti C, Cobanaj M, Marian F, et al. Artificial intelligence for prediction of treatment outcomes in breast cancer: Systematic review of design, reporting standards, and bias. Cancer Treat Rev. 2022;108:102410.
-
17.
Deliwala SS, Hamid K, Barbarawi M, et al. Artificial intelligence (AI) real-time detection vs. routine colonoscopy for colorectal neoplasia: a meta-analysis and trial sequential analysis. Int J Colorectal Dis. 2021;36(11):2291-2303.
-
18.
Dumitrescu EA, Ungureanu BS, Cazacu IM, et al. Diagnostic Value of Artificial Intelligence-Assisted Endoscopic Ultrasound for Pancreatic Cancer: A Systematic Review and Meta-Analysis. Diagnostics (Basel). 2022;12(2).
-
19.
Fatania K, Mohamud F, Clark A, et al. Intensity standardization of MRI prior to radiomic feature extraction for artificial intelligence research in glioma-a systematic review. Eur Radiol. 2022;32(10):7014-7025.
-
20.
Ferrante di Ruffano L, Takwoingi Y, Dinnes J, et al. Computer-assisted diagnosis techniques (dermoscopy and spectroscopy-based) for diagnosing skin cancer in adults. Cochrane Database Syst Rev. 2018;12(12):Cd013186.
-
21.
Freeman K, Geppert J, Stinton C, et al. Use of artificial intelligence for image analysis in breast cancer screening programmes: systematic review of test accuracy. Bmj. 2021;374:n1872.
-
22.
Hassan C, Spadaccini M, Iannone A, et al. Performance of artificial intelligence in colonoscopy for adenoma and polyp detection: a systematic review and meta-analysis. Gastrointest Endosc. 2021;93(1):77-85.e76.
-
23.
Hickman SE, Woitek R, Le EPV, et al. Machine Learning for Workflow Applications in Screening Mammography: Systematic Review and Meta-Analysis. Radiology. 2022;302(1):88-104.
-
24.
Huang G, Wei X, Tang H, Bai F, Lin X, Xue D. A systematic review and meta-analysis of diagnostic performance and physicians' perceptions of artificial intelligence (AI)-assisted CT diagnostic technology for the classification of pulmonary nodules. J Thorac Dis. 2021;13(8):4797-4811.
-
25.
Jiang K, Jiang X, Pan J, et al. Current Evidence and Future Perspective of Accuracy of Artificial Intelligence Application for Early Gastric Cancer Diagnosis With Endoscopy: A Systematic and Meta-Analysis. Front Med (Lausanne). 2021;8:629080.
-
26.
Jones OT, Calanzani N, Saji S, et al. Artificial Intelligence Techniques That May Be Applied to Primary Care Data to Facilitate Earlier Diagnosis of Cancer: Systematic Review. J Med Internet Res. 2021;23(3):e23483.
-
27.
Jones OT, Matin RN, van der Schaar M, et al. Artificial intelligence and machine learning algorithms for early detection of skin cancer in community and primary care settings: a systematic review. Lancet Digit Health. 2022;4(6):e466-e476.
-
28.
Khanagar SB, Naik S, Al Kheraif AA, et al. Application and Performance of Artificial Intelligence Technology in Oral Cancer Diagnosis and Prediction of Prognosis: A Systematic Review. Diagnostics (Basel). 2021;11(6).
-
29.
Kim HY, Cho SJ, Sunwoo L, et al. Classification of true progression after radiotherapy of brain metastasis on MRI using artificial intelligence: a systematic review and meta-analysis. Neurooncol Adv. 2021;3(1):vdab080.
-
30.
Klarenbeek SE, Weekenstroo HHA, Sedelaar JPM, Fütterer JJ, Prokop M, Tummers M. The Effect of Higher Level Computerized Clinical Decision Support Systems on Oncology Care: A Systematic Review. Cancers (Basel). 2020;12(4).
-
31.
Kozikowski M, Suarez-Ibarrola R, Osiecki R, et al. Role of Radiomics in the Prediction of Muscle-invasive Bladder Cancer: A Systematic Review and Meta-analysis. Eur Urol Focus. 2022;8(3):728-738.
-
32.
Lai Q, Spoletini G, Mennini G, et al. Prognostic role of artificial intelligence among patients with hepatocellular cancer: A systematic review. World J Gastroenterol. 2020;26(42):6679-6688.
-
33.
Lu SC, Xu C, Nguyen CH, Geng Y, Pfob A, Sidey-Gibbons C. Machine Learning-Based Short-Term Mortality Prediction Models for Patients With Cancer Using Electronic Health Record Data: Systematic Review and Critical Appraisal. JMIR Med Inform. 2022;10(3):e33182.
-
34.
Mahmood H, Shaban M, Indave BI, Santos-Silva AR, Rajpoot N, Khurram SA. Use of artificial intelligence in diagnosis of head and neck precancerous and cancerous lesions: A systematic review. Oral Oncol. 2020;110:104885.
-
35.
Marka A, Carter JB, Toto E, Hassanpour S. Automated detection of nonmelanoma skin cancer using digital images: a systematic review. BMC Med Imaging. 2019;19(1):21.
-
36.
Mühlbauer J, Egen L, Kowalewski KF, et al. Radiomics in Renal Cell Carcinoma-A Systematic Review and Meta-Analysis. Cancers (Basel). 2021;13(6).
-
37.
Nazarian S, Glover B, Ashrafian H, Darzi A, Teare J. Diagnostic Accuracy of Artificial Intelligence and Computer-Aided Diagnosis for the Detection and Characterization of Colorectal Polyps: Systematic Review and Meta-analysis. J Med Internet Res. 2021;23(7):e27370.
-
38.
Ng WT, But B, Choi HCW, et al. Application of Artificial Intelligence for Nasopharyngeal Carcinoma Management - A Systematic Review. Cancer Manag Res. 2022;14:339-366.
-
39.
Ninatti G, Kirienko M, Neri E, Sollini M, Chiti A. Imaging-Based Prediction of Molecular Therapy Targets in NSCLC by Radiogenomics and AI Approaches: A Systematic Review. Diagnostics (Basel). 2020;10(6).
-
40.
Park JH, Kim EY, Luchini C, et al. Artificial Intelligence for Predicting Microsatellite Instability Based on Tumor Histomorphology: A Systematic Review. Int J Mol Sci. 2022;23(5).
-
41.
Partouche E, Yeh R, Eche T, et al. Updated Trends in Imaging Practices for Pancreatic Neuroendocrine Tumors (PNETs): A Systematic Review and Meta-Analysis to Pave the Way for Standardization in the New Era of Big Data and Artificial Intelligence. Front Oncol. 2021;11:628408.
-
42.
Popescu ER, Geantă M, Brand A. Mapping of clinical research on artificial intelligence in the treatment of cancer and the challenges and opportunities underpinning its integration in the European Union health sector. Eur J Public Health. 2022;32(3):443-449.
-
43.
Prasoppokakorn T, Tiyarattanachai T, Chaiteerakij R, et al. Application of artificial intelligence for diagnosis of pancreatic ductal adenocarcinoma by EUS: A systematic review and meta-analysis. Endosc Ultrasound. 2022;11(1):17-26.
-
44.
Qin K, Li J, Fang Y, et al. Convolution neural network for the diagnosis of wireless capsule endoscopy: a systematic review and meta-analysis. Surg Endosc. 2022;36(1):16-31.
-
45.
Ravegnini G, Ferioli M, Morganti AG, et al. Radiomics and Artificial Intelligence in Uterine Sarcomas: A Systematic Review. J Pers Med. 2021;11(11).
-
46.
Rezayi S, S RNK, Saeedi S. Effectiveness of Artificial Intelligence for Personalized Medicine in Neoplasms: A Systematic Review. Biomed Res Int. 2022;2022:7842566.
-
47.
Staal FCR, van der Reijd DJ, Taghavi M, Lambregts DMJ, Beets-Tan RGH, Maas M. Radiomics for the Prediction of Treatment Outcome and Survival in Patients With Colorectal Cancer: A Systematic Review. Clin Colorectal Cancer. 2021;20(1):52-71.
-
48.
Sushentsev N, Moreira Da Silva N, Yeung M, et al. Comparative performance of fully-automated and semi-automated artificial intelligence methods for the detection of clinically significant prostate cancer on MRI: a systematic review. Insights Imaging. 2022;13(1):59.
-
49.
Syer T, Mehta P, Antonelli M, et al. Artificial Intelligence Compared to Radiologists for the Initial Diagnosis of Prostate Cancer on Magnetic Resonance Imaging: A Systematic Review and Recommendations for Future Studies. Cancers (Basel). 2021;13(13).
-
50.
Tohidinezhad F, Pennetta F, van Loon J, Dekker A, de Ruysscher D, Traverso A. Prediction models for treatment-induced cardiac toxicity in patients with non-small-cell lung cancer: A systematic review and meta-analysis. Clin Transl Radiat Oncol. 2022;33:134-144.
-
51.
Visaggi P, Barberio B, Gregori D, et al. Systematic review with meta-analysis: artificial intelligence in the diagnosis of oesophageal diseases. Aliment Pharmacol Ther. 2022;55(5):528-540.
-
52.
Wessels F, Kuntz S, Krieghoff-Henning E, et al. Artificial intelligence to predict oncological outcome directly from hematoxylin and eosin-stained slides in urology. Minerva Urol Nephrol. 2022;74(5):538-550.
-
53.
Xu L, Sanders L, Li K, Chow JCL. Chatbot for Health Care and Oncology Applications Using Artificial Intelligence and Machine Learning: Systematic Review. JMIR Cancer. 2021;7(4):e27850.
-
54.
Xu Y, Ding W, Wang Y, et al. Comparison of diagnostic performance between convolutional neural networks and human endoscopists for diagnosis of colorectal polyp: A systematic review and meta-analysis. PLoS One. 2021;16(2):e0246892.
-
55.
Yin H, Yang X, Sun L, et al. The value of artificial intelligence techniques in predicting pancreatic ductal adenocarcinoma with EUS images: A meta-analysis and systematic review. Endosc Ultrasound. 2022.
-
56.
Yung A, Kay J, Beale P, Gibson KA, Shaw T. Computer-Based Decision Tools for Shared Therapeutic Decision-making in Oncology: Systematic Review. JMIR Cancer. 2021;7(4):e31616.
-
57.
Zhang SM, Wang YJ, Zhang ST. Accuracy of artificial intelligence-assisted detection of esophageal cancer and neoplasms on endoscopic images: A systematic review and meta-analysis. J Dig Dis. 2021;22(6):318-328.
-
58.
Zhao Y, Hu B, Wang Y, Yin X, Jiang Y, Zhu X. Identification of gastric cancer with convolutional neural networks: a systematic review. Multimed Tools Appl. 2022;81(8):11717-11736.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Taber, P., Armin, J.S., Orozco, G. et al. Artificial Intelligence and Cancer Control: Toward Prioritizing Justice, Equity, Diversity, and Inclusion (JEDI) in Emerging Decision Support Technologies. Curr Oncol Rep 25, 387–424 (2023). https://doi.org/10.1007/s11912-023-01376-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-023-01376-7