Abstract
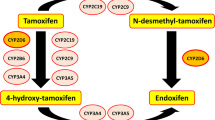
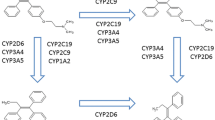
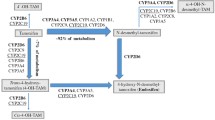
The selective estrogen receptor modulator tamoxifen has been used for more than three decades to treat metastatic and early-stage receptor-positive breast cancer and, more recently, to prevent the disease. Biotransformation of tamoxifen to the potent antiestrogen endoxifen is performed by cytochrome P450 (CYP) enzymes, in particular the CYP2D6 isoform. Genetic variants in the CYP2D6 gene may result in CYP2D6 enzymes with reduced or null activity. Strong and intermediate inhibitors of CYP2D6, which may be used to treat hot flashes or psychiatric conditions in breast cancer patients, can also negatively impact enzyme function. Prospective data are lacking, but the balance of current evidence strongly suggests that, compared with women with two wild-type alleles, the presence of two null alleles, and possibly one null allele, predicts reduced tamoxifen metabolism and an inferior outcome in postmenopausal women with early breast cancer who receive adjuvant treatment with the drug. Unfortunately, studies to date have been largely retrospective and the interpretation of their results is limited by examination of archival tissue samples and the inclusion of heterogeneous populations. Although we do not currently recommend routine CYP2D6 testing for women who do not have alternative standard therapies, the use of concomitant strong or intermediate inhibitors of CYP2D6 should be avoided if feasible. This review summarizes the literature to date with a focus on clinically relevant recent studies that examined the association between CYP2D6 polymorphisms and tamoxifen-associated outcomes.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Jemal A, Siegel R, Ward E, et al.: Cancer statistics, 2009. CA Cancer J Clin 2009, 59:225–249.
Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005, 365:1687–1717.
Guttmacher AE, Collins FS: Welcome to the genomic era. N Engl J Med 2003, 349:996–998.
Weinshilboum R: Inheritance and drug response. N Engl J Med 2003, 348:529–537.
Evans WE, McLeod HL: Pharmacogenomics—drug disposition, drug targets, and side effects. N Engl J Med 2003, 348:538–549.
• Desta Z, Ward BA, Soukhova NV, Flockhart DA: Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther 2004, 310:1062–1075. This article offers a thorough explanation of the metabolism pathway of tamoxifen outlining the contribution of CYP450 enzymes.
Fabian C, Tilzer L, Sternson L: Comparative binding affinities of tamoxifen, 4-hydroxytamoxifen, and desmethyltamoxifen for estrogen receptors isolated from human breast carcinoma: correlation with blood levels in patients with metastatic breast cancer. Biopharm Drug Dispos 1981, 2:381–390.
Johnson MD, Zuo H, Lee KH, et al.: Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat. 2004, 85:151–159.
Lim YC, Li L, Desta Z, et al.: Endoxifen, a secondary metabolite of tamoxifen, and 4-OH-tamoxifen induce similar changes in global gene expression patterns in MCF-7 breast cancer cells. J Pharmacol Exp Ther 2006, 318:503–512.
Stearns V, Johnson MD, Rae JM, et al.: Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst 2003, 95:1758–1764.
Wu X, Hawse JR, Subramaniam M, et al.: The tamoxifen metabolite, endoxifen, is a potent antiestrogen that targets estrogen receptor alpha for degradation in breast cancer cells. Cancer Res 2009, 69:1722–1727.
Borges S, Desta Z, Li L, et al.: Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatment. Clin Pharmacol Ther 2006, 80:61–74.
Sun D, Sharma AK, Dellinger RW, et al.: Glucuronidation of active tamoxifen metabolites by the human UDP glucuronosyltransferases. Drug Metab Dispos 2007, 35:2006–2014.
Nowell S, Sweeney C, Winters M, et al.: Association between sulfotransferase 1A1 genotype and survival of breast cancer patients receiving tamoxifen therapy. J Natl Cancer Inst 2002, 94:1635–1640.
Nowell SA, Ahn J, Rae JM, et al.: Association of genetic variation in tamoxifen-metabolizing enzymes with overall survival and recurrence of disease in breast cancer patients. Breast Cancer Res Treat 2005, 91:249–258.
Rodriguez-Antona C, Ingelman-Sundberg M: Cytochrome P450 pharmacogenetics and cancer. Oncogene 2006, 25:1679–1691.
Home page of the Human cytochrome P450 (CYP) Allele Nomenclature. Edited by Ingelman-Sundberg M, Daly AK, Nebert DW. Available at http://www.cypalleles.ki.se. Accessed November 23, 2009.
Bradford LD: CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics 2002, 3:229–243.
Garte S, Gaspari L, Alexandrie AK, et al.: Metabolic gene polymorphism frequencies in control populations. Cancer Epidemiol Biomarkers Prev 2001, 10:1239–1248.
Ingelman-Sundberg M: Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogenomics J 2005, 5:6–13.
Gaedigk A, Bradford LD, Marcucci KA, Leeder JS: Unique CYP2D6 activity distribution and genotype-phenotype discordance in black Americans. Clin Pharmacol Ther 2002, 72:76–89.
Dahl ML, Johansson I, Bertilsson L, et al.: Ultrarapid hydroxylation of debrisoquine in a Swedish population. Analysis of the molecular genetic basis. J Pharmacol Exp Ther 1995, 274:516–520.
Alfaro CL, Lam YW, Simpson J, Ereshefsky L: CYP2D6 inhibition by fluoxetine, paroxetine, sertraline, and venlafaxine in a crossover study: intraindividual variability and plasma concentration correlations. J Clin Pharmacol 2000, 40:58–66.
Jin Y, Desta Z, Stearns V, et al.: CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst 2005, 97:30–39.
Ahern TP, Pedersen L, Cronin-Fenton DP, et al.: No increase in breast cancer recurrence with concurrent use of tamoxifen and some CYP2D6-inhibiting medications. Cancer Epidemiol Biomarkers Prev 2009, 18:2562–2564.
Aubert RE, Stanek EJ, Yao J, et al.: Risk of breast cancer recurrence in women initiating tamoxifen with CYP2D6 inhibitors [abstract]. J Clin Oncol 2009, 28:508.
Dezentje VO, Van Blijderveen NJ, Gelderblom H, et al.: Concomitant CYP2D6 inhibitor use and tamoxifen adherence in early-stage breast cancer: a pharmacoepidemiologic study [abstract]. J Clin Oncol 2009, 27:509.
Ingle JN, Suman VJ, Mailliard JA, et al.: Randomized trial of tamoxifen alone or combined with fluoxymesterone as adjuvant therapy in postmenopausal women with resected estrogen receptor positive breast cancer. North Central Cancer Treatment Group Trial 89-30–52. Breast Cancer Res Treat 2006, 98:217–222.
• Goetz MP, Rae JM, Suman VJ, et al.: Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol 2005, 23:9312–9318. This article offers the first retrospective study of the relationship between CYP2D6*4 status and clinical outcome in 256 women receiving adjuvant tamoxifen. Recurrence-free survival was significantly reduced among CYP2D6*4/*4 patients relative to patients with wild-type genotype.
Goetz MP, Knox SK, Suman VJ, et al.: The impact of cytochrome P450 2D6 metabolism in women receiving adjuvant tamoxifen. Breast Cancer Res Treat 2007, 101:113–121.
Wegman P, Vainikka L, Stal O, et al.: Genotype of metabolic enzymes and the benefit of tamoxifen in postmenopausal breast cancer patients. Breast Cancer Res 2005, 7:R284–R290.
Wegman P, Elingarami S, Carstensen J, et al.: Genetic variants of CYP3A5, CYP2D6, SULT1A1, UGT2B15 and tamoxifen response in postmenopausal patients with breast cancer. Breast Cancer Res 2007, 9:R7.
Schroth W, Antoniadou L, Fritz P, et al.: Breast cancer treatment outcome with adjuvant tamoxifen relative to patient CYP2D6 and CYP2C19 genotypes. J Clin Oncol 2007, 25:5187–5193.
Lim HS, Ju Lee H, Seok Lee K, et al.: Clinical implications of CYP2D6 genotypes predictive of tamoxifen pharmacokinetics in metastatic breast cancer. J Clin Oncol 2007, 25:3837–3845.
Kiyotani K, Mushiroda T, Sasa M, et al.: Impact of CYP2D6*10 on recurrence-free survival in breast cancer patients receiving adjuvant tamoxifen therapy. Cancer Sci 2008, 99:995–999.
Xu Y, Sun Y, Yao L, et al.: Association between CYP2D6 *10 genotype and survival of breast cancer patients receiving tamoxifen treatment. Ann Oncol 2008, 19:1423–1429.
Ramon YCT, Altes A, Pare L, et al.: Impact of CYP2D6 polymorphisms in tamoxifen adjuvant breast cancer treatment. Breast Cancer Res Treat 2009 Feb 3 (Epub ahead of print).
• Schroth W, Goetz MP, Hamann U, et al.: Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. JAMA 2009, 302:1429–1436. This article discusses a recent combined retrospective and prospective study of 1325 patients treated with tamoxifen in the adjuvant setting. The investigators reported a significantly reduced recurrence-free survival in intermediate or poor metabolizers of tamoxifen compared with extensive metabolizers.
Okishiro M, Taguchi T, Jin Kim S, et al.: Genetic polymorphisms of CYP2D6 10 and CYP2C19 2, 3 are not associated with prognosis, endometrial thickness, or bone mineral density in Japanese breast cancer patients treated with adjuvant tamoxifen. Cancer 2009, 115:952–961.
Bijl MJ, van Schaik RH, Lammers LA, et al.: The CYP2D6*4 polymorphism affects breast cancer survival in tamoxifen users. Breast Cancer Res Treat 2009, 118:125–130.
• Lash TL, Lien EA, Sorensen HT, Hamilton-Dutoit S: Genotype-guided tamoxifen therapy: time to pause for reflection? Lancet Oncol 2009, 10:825–833. This article provides commentary describing the controversy regarding the adoption of routine CYP2D6 testing in the clinic.
Narod SA, Brunet JS, Ghadirian P, et al.: Tamoxifen and risk of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers: a case-control study. Hereditary Breast Cancer Clinical Study Group. Lancet 2000, 356:1876–1881.
Gronwald J, Tung N, Foulkes WD, et al.: Tamoxifen and contralateral breast cancer in BRCA1 and BRCA2 carriers: an update. Int J Cancer 2006, 118:2281–2284.
Newman WG, Hadfield KD, Latif A, et al.: Impaired tamoxifen metabolism reduces survival in familial breast cancer patients. Clin Cancer Res 2008, 14:5913–5918.
Bonanni B, Macis D, Maisonneuve P, et al.: Polymorphism in the CYP2D6 tamoxifen-metabolizing gene influences clinical effect but not hot flashes: data from the Italian Tamoxifen Trial. J Clin Oncol 2006, 24:3708–3709; author reply 3709.
Lynn Henry N, Rae JM, Li L, et al.: Association between CYP2D6 genotype and tamoxifen-induced hot flashes in a prospective cohort. Breast Cancer Res Treat 2009, 117:571–575.
Rae JM, Sikora MJ, Henry NL, et al.: Cytochrome P450 2D6 activity predicts discontinuation of tamoxifen therapy in breast cancer patients. Pharmacogenomics J 2009, 9:258–264.
Jin Y, Hayes DF, Li L, et al.: Estrogen receptor genotypes influence hot flash prevalence and composite score before and after tamoxifen therapy. J Clin Oncol 2008, 26:5849–5854.
Zhang QX, Borg A, Wolf DM, et al.: An estrogen receptor mutant with strong hormone-independent activity from a metastatic breast cancer. Cancer Res 1997, 57:1244–1249.
Baum M, Budzar AU, Cuzick J, et al.: Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet 2002, 359:2131–2139.
Coombes RC, Hall E, Gibson LJ, et al.: A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 2004, 350:1081–1092.
Thurlimann B, Keshaviah A, Coates AS, et al.: A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 2005, 353:2747–2757.
Punglia RS, Burstein HJ, Winer EP, Weeks JC: Pharmacogenomic variation of CYP2D6 and the choice of optimal adjuvant endocrine therapy for postmenopausal breast cancer: a modeling analysis. J Natl Cancer Inst 2008, 100:642–648.
Ma CX, Adjei AA, Salavaggione OE, et al.: Human aromatase: gene resequencing and functional genomics. Cancer Res 2005, 65:11071–11082.
Colomer R, Monzo M, Tusquets I, et al.: A single-nucleotide polymorphism in the aromatase gene is associated with the efficacy of the aromatase inhibitor letrozole in advanced breast carcinoma. Clin Cancer Res 2008, 14:811–816.
Connolly R, Stearns V: The role of pharmacogenetics in selection of breast cancer treatment. Current Breast Cancer Reports 2009, 1:190–197.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Higgins, M.J., Stearns, V. CYP2D6 Polymorphisms and Tamoxifen Metabolism: Clinical Relevance. Curr Oncol Rep 12, 7–15 (2010). https://doi.org/10.1007/s11912-009-0076-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-009-0076-5