Summary
Purpose
This clinical trial evaluated the addition of fluoxymesterone (Flu) to tamoxifen (Tam) in women with resected early stage breast cancer and attempted to corroborate the findings of superiority for the combination over Tam alone seen in a previous randomized trial in metastatic disease.
Patients and methods
Postmenopausal women with early stage breast cancer that was known to be estrogen receptor (ER) positive were randomized to treatment with Tam (20 mg per day orally for 5 years) alone or combined with Flu (10 mg orally twice per day for 1 year). The primary endpoint was relapse-free survival (RFS) defined as local-regional or distant recurrence including ductal carcinoma in situ of the ipsilateral, but not contralateral breast, and death from any cause.
Results
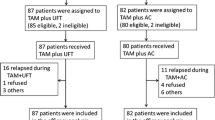
There were 541 eligible patients entered between 1991 and 1995 and the treatment arms were balanced with respect to patient characteristics. The median follow up of patients still alive was 11.4 years. No significant difference was found between Tam plus Flu and Tam alone in terms of RFS or overall survival. The adjusted hazard ratio (Tam+Flu/Tam) for relapse or death without relapse was estimated to be 0.84 (95% CI: 0.64–1.10) and that for death was 0.89 (95% CI: 0.67–1.18). As expected there was more virilization in women who received Flu.
Conclusions
This clinical trial did not demonstrate superiority of Tam plus Flu over Tam alone as adjuvant therapy for postmenopausal women with resected early breast cancer known to be ER positive.
Similar content being viewed by others
References
Ingle JN, Twito DI, Schaid DJ, et al. Combination hormonal therapy with tamoxifen plus fluoxymesterone versus tamoxifen alone in postmenopausal women with metastatic breast cancer, an updated analysis Cancer 67:886–891, 1991
Tormey DC, Lippman ME, Edwards BK, et al. Evaluation of tamoxifen doses with and without fluoxymesterone in advanced breast cancer Ann Int Med 98:139–144, 1983
Lippman M, Huff K A demonstration of androgen and estrogen receptors in a human breast cancer using a new protamine sulfate assay Cancer 38:868–874, 1976
American Joint Committee on Cancer: Manual for Staging of Cancer. Third edition. In: Beahrs OH, Henson DE, Hutter RVP, Myers MH (eds) J.B. Lippincott, Philadelphia, 1988, pp. 145–150
Moinfar F, Okcu M, Tsybrovskyy O, et al. Androgen receptors frequently are expressed in breast cancinomas Cancer 98:703–711, 2003
Kennedy BJ Fluoxymesterone therapy in advanced breast cancer N Engl J Med 259:673–675, 1958
Adair FE, Hermann JB The use of testosterone proprionate in the treatment of advanced carcinoma of the breast Ann Surg 123:1023–1035, 1946
Rose C, Kamby C, Mouridsen HT, et al. Combined endocrine treatment of elderly postmenopausal patients with metastatic breast cancer Breast Cancer Res Treat 61:103–110, 2000
Acknowledgements
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported in part by Public Health Service grants CA-25224, CA-37404, CA-15083, CA-35113, CA-35269, CA-63849, CA-35103, CA-35195, CA-35272, CA-35101, CA-37417, CA-35415, CA-52352, CA-35448, CA-60276. Additional participating institutions include: Meritcare Hospital CCOP, Fargo, ND 58122 (Ralph Levitt, M.D.); Toledo Community Hospital Oncology Program CCOP, Toledo, OH 43610 (Paul L. Schaefer, M.D.); Cedar Rapids Oncology Project CCOP, Cedar Rapids, IA 52403 (Martin Wiesenfeld, M.D.); Quain and Ramstad Clinic, Bismarck, ND 58506 (Edward Wos, D.O.); CentraCare Clinic, St. Cloud, MN 56301 (Harold E. Windschitl, M.D.); Altru Health Systems, Grand Forks, ND 58201 (Tudor Dentchev, M.D.); Sioux Community Cancer Consortium, Sioux Falls, SD 57105 (Loren K. Tschetter, M.D.); Saskatchewan Cancer Foundation, CANADA S7N 4H4 (Muhammad Salim, M.D.); Rapid City Regional Oncology Group, Rapid City, SD 59709 (Larry P. Ebbert, M.D.); Scottsdale CCOP, Scottsdale, AZ 8525 (Tom Fitch, M.D.); Carle Cancer Center CCOP, Urbana, IL 61801 (Kendrith Rowland, M.D); Ochsner Community Clinical Oncology Program, New Orleans, LA 70121 (Carl G. Kardinal, M.D.); Iowa Oncology Research Association CCOP, Des Moines, IA 50309-1014 (Roscoe F. Morton, M.D.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ingle, J.N., Suman, V.J., Mailliard, J.A. et al. Randomized trial of tamoxifen alone or combined with fluoxymesterone as adjuvant therapy in postmenopausal women with resected estrogen receptor positive breast cancer. North Central Cancer Treatment Group Trial 89-30-52. Breast Cancer Res Treat 98, 217–222 (2006). https://doi.org/10.1007/s10549-005-9152-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-005-9152-1