Abstract
Purpose of Review
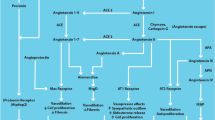
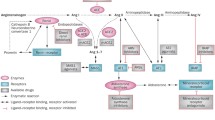
The renin-angiotensin-aldosterone system (RAAS) plays important roles in regulating blood pressure and body fluid, which contributes to the pathophysiology of hypertension and cardiovascular/renal diseases. However, accumulating evidence has further revealed the complexity of this signal transduction system, including direct interactions with other receptors and proteins. This review focuses on recent research advances in RAAS with an emphasis on its receptors.
Recent Findings
Both systemically and locally produced angiotensin II (Ang II) bind to Ang II type 1 receptor (AT1R) and elicit strong biological functions. Recent studies have shown that Ang II–induced activation of Ang II type 2 receptor (AT2R) elicits the opposite functions to those of AT1R. However, accumulating evidence has now expanded the components of RAAS, including (pro)renin receptor, angiotensin-converting enzyme 2, angiotensin 1–7, and Mas receptor. In addition, the signal transductions of AT1R and AT2R are regulated by not only Ang II but also its receptor-associated proteins such as AT1R-associated protein and AT2R-interacting protein. Recent studies have indicated that inappropriate activation of local mineralocorticoid receptor contributes to cardiovascular and renal tissue injuries through aldosterone-dependent and -independent mechanisms.
Summary
Since the mechanisms of RAAS signal transduction still remain to be elucidated, further investigations are necessary to explore novel molecular mechanisms of the RAAS, which will provide alternative therapeutic agents other than existing RAAS blockers.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52(3):415–72.
Padia SH, Carey RM. AT2 receptors: beneficial counter-regulatory role in cardiovascular and renal function. Arch Eur J Physiol. 2013;465(1):99–110. https://doi.org/10.1007/s00424-012-1146-3.
•• Forrester SJ, Booz GW, Sigmund CD, Coffman TM, Kawai T, Rizzo V, et al. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol Rev. 2018;98(3):1627–738. https://doi.org/10.1152/physrev.00038.2017This paper summarizes detailed mechanisms of the angiotensin II signal transduction in various tissues.
Nishiyama A. Pathophysiological mechanisms of mineralocorticoid receptor-dependent cardiovascular and chronic kidney disease. Hypertens Res. 2019;42(3):293–300. https://doi.org/10.1038/s41440-018-0158-6.
Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109(11):1417–27. https://doi.org/10.1172/JCI14276.
Suzuki F, Hayakawa M, Nakagawa T, Nasir UM, Ebihara A, Iwasawa A, et al. Human prorenin has "gate and handle" regions for its non-proteolytic activation. J Biol Chem. 2003;278(25):22217–22. https://doi.org/10.1074/jbc.M302579200.
Ichihara A, Yatabe MS. The (pro)renin receptor in health and disease. Nat Rev Nephrol. 2019;15:693–712. https://doi.org/10.1038/s41581-019-0160-5.
Cousin C, Bracquart D, Contrepas A, Corvol P, Muller L, Nguyen G. Soluble form of the (pro)renin receptor generated by intracellular cleavage by furin is secreted in plasma. Hypertension. 2009;53(6):1077–82. https://doi.org/10.1161/HYPERTENSIONAHA.108.127258.
Yoshikawa A, Aizaki Y, Kusano K, Kishi F, Susumu T, Iida S, et al. The (pro)renin receptor is cleaved by ADAM19 in the Golgi leading to its secretion into extracellular space. Hypertens Res. 2011;34(5):599–605. https://doi.org/10.1038/hr.2010.284.
Forgac M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol. 2007;8(11):917–29. https://doi.org/10.1038/nrm2272.
Ludwig J, Kerscher S, Brandt U, Pfeiffer K, Getlawi F, Apps DK, et al. Identification and characterization of a novel 9.2-kDa membrane sector-associated protein of vacuolar proton-ATPase from chromaffin granules. J Biol Chem. 1998;273(18):10939–47. https://doi.org/10.1074/jbc.273.18.10939.
Kanda A, Noda K, Ishida S. ATP6AP2/(pro)renin receptor contributes to glucose metabolism via stabilizing the pyruvate dehydrogenase E1 beta subunit. J Biol Chem. 2015;290(15):9690–700. https://doi.org/10.1074/jbc.M114.626713.
Peters J. The (pro)renin receptor and its interaction partners. Arch Eur J Physiol. 2017;469(10):1245–56. https://doi.org/10.1007/s00424-017-2005-z.
•• Buechling T, Bartscherer K, Ohkawara B, Chaudhary V, Spirohn K, Niehrs C, et al. Wnt/Frizzled signaling requires dPRR, the Drosophila homolog of the prorenin receptor. Curr Biol. 2010;20(14):1263–8. https://doi.org/10.1016/j.cub.2010.05.028In this paper, genome-wide screening studies have demonstrated that PRR is an essential component for the regulation of canonical Wnt/β-catenin signaling pathway.
Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4(2):68–75. https://doi.org/10.4161/org.4.2.5851.
Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, et al. Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science. 2010;327(5964):459–63. https://doi.org/10.1126/science.1179802.
Hermle T, Guida MC, Beck S, Helmstadter S, Simons M. Drosophila ATP6AP2/VhaPRR functions both as a novel planar cell polarity core protein and a regulator of endosomal trafficking. EMBO J. 2013;32(2):245–59. https://doi.org/10.1038/emboj.2012.323.
• Shibayama Y, Fujimori T, Nguyen G, Hirose T, Totsune K, Ichihara A, et al. (Pro)renin receptor is crucial for Wnt/beta-catenin-dependent genesis of pancreatic ductal adenocarcinoma. Sci Rep. 2015;5:8854. https://doi.org/10.1038/srep08854In this paper, the authors identified aberrant expression of PRR in pancreatic ductal adenocarcinoma (PDAC) tissues. Furthermore, plasma soluble PRR levels were significantly increased in patients with PDAC. Further molecular studies revealed new critical aspects that strongly indicate a potential role of (P)RR in Wnt/β-catenin signaling-dependent genesis of PDAC.
Arundhathi A, Chuang WH, Chen JK, Wang SE, Shyr YM, Chen JY, et al. Prorenin receptor acts as a potential molecular target for pancreatic ductal adenocarcinoma diagnosis. Oncotarget. 2016;7(34):55437–48. https://doi.org/10.18632/oncotarget.10583.
Kouchi M, Shibayama Y, Ogawa D, Miyake K, Nishiyama A, Tamiya T. (Pro)renin receptor is crucial for glioma development via the Wnt/beta-catenin signaling pathway. J Neurosurg. 2017;127(4):819–28. https://doi.org/10.3171/2016.9.JNS16431.
Ohba K, Suzuki T, Nishiyama H, Kaneko K, Hirose T, Totsune K, et al. Expression of (pro)renin receptor in breast cancers and its effect on cancercell proliferation. Biomed Res. 2014;35(2):117–26.
Beitia M, Solano-Iturri JD, Errarte P, Calvete-Candenas J, Loizate A, Etxezarraga MC, et al. (Pro)renin receptor expression increases throughout the colorectal adenoma-adenocarcinoma sequence and it is associated with worse colorectal cancer prognosis. Cancers (Basel). 2019;11(6):881. https://doi.org/10.3390/cancers11060881.
Wang J, Shibayama Y, Zhang A, Ohsaki H, Asano E, Suzuki Y, et al. (Pro)renin receptor promotes colorectal cancer through the Wnt/beta-catenin signalling pathway despite constitutive pathway component mutations. Br J Cancer. 2019;120(2):229–37. https://doi.org/10.1038/s41416-018-0350-0.
Yamamoto H, Kaneko K, Ohba K, Morimoto R, Hirose T, Satoh F, et al. Increased expression of (pro)renin receptor in aldosterone-producing adenomas. Peptides. 2013;49:68–73. https://doi.org/10.1016/j.peptides.2013.08.022.
Montezano AC, Nguyen Dinh Cat A, Rios FJ, Touyz RM. Angiotensin II and vascular injury. Curr Hypertens Rep. 2014;16(6):431. https://doi.org/10.1007/s11906-014-0431-2.
Higuchi S, Ohtsu H, Suzuki H, Shirai H, Frank GD, Eguchi S. Angiotensin II signal transduction through the AT1 receptor: novel insights into mechanisms and pathophysiology. Clin Sci. 2007;112(8):417–28. https://doi.org/10.1042/CS20060342.
Kamo T, Akazawa H, Komuro I. Pleiotropic effects of angiotensin II receptor signaling in cardiovascular homeostasis and aging. Int Heart J. 2015;56(3):249–54. https://doi.org/10.1536/ihj.14-429.
Toth AD, Turu G, Hunyady L, Balla A. Novel mechanisms of G-protein-coupled receptors functions: AT1 angiotensin receptor acts as a signaling hub and focal point of receptor cross-talk. Best Pract Res Clin Endocrinol Metab. 2018;32(2):69–82. https://doi.org/10.1016/j.beem.2018.02.003.
Ali MS, Sayeski PP, Dirksen LB, Hayzer DJ, Marrero MB, Bernstein KE. Dependence on the motif YIPP for the physical association of Jak2 kinase with the intracellular carboxyl tail of the angiotensin II AT1 receptor. J Biol Chem. 1997;272(37):23382–8. https://doi.org/10.1074/jbc.272.37.23382.
Venema RC, Ju H, Venema VJ, Schieffer B, Harp JB, Ling BN, et al. Angiotensin II-induced association of phospholipase Cgamma1 with the G-protein-coupled AT1 receptor. J Biol Chem. 1998;273(13):7703–8. https://doi.org/10.1074/jbc.273.13.7703.
Tamura K, Tanaka Y, Tsurumi Y, Azuma K, Shigenaga A, Wakui H, et al. The role of angiotensin AT1 receptor-associated protein in renin-angiotensin system regulation and function. Curr Hypertens Rep. 2007;9(2):121–7.
Tamura K, Wakui H, Maeda A, Dejima T, Ohsawa M, Azushima K, et al. The physiology and pathophysiology of a novel angiotensin receptor-binding protein ATRAP/Agtrap. Curr Pharm Des. 2013;19(17):3043–8.
• Tamura K, Wakui H, Azushima K, Uneda K, Haku S, Kobayashi R, et al. Angiotensin II type 1 receptor binding molecule ATRAP as a possible modulator of renal sodium handling and blood pressure in pathophysiology. Curr Med Chem. 2015;22(28):3210–6 Together with references 31 and 32, these papers summarize physiology and pathophysiology of angiotensin II type 1 receptor-associated protein (ATRAP) in renin-angiotensin system and blood pressure regulation.
Wakui H, Tamura K, Tanaka Y, Matsuda M, Bai Y, Dejima T, et al. Cardiac-specific activation of angiotensin II type 1 receptor-associated protein completely suppresses cardiac hypertrophy in chronic angiotensin II-infused mice. Hypertension. 2010;55(5):1157–64. https://doi.org/10.1161/HYPERTENSIONAHA.109.147207.
Wakui H, Dejima T, Tamura K, Uneda K, Azuma K, Maeda A, et al. Activation of angiotensin II type 1 receptor-associated protein exerts an inhibitory effect on vascular hypertrophy and oxidative stress in angiotensin II-mediated hypertension. Cardiovasc Res. 2013;100(3):511–9. https://doi.org/10.1093/cvr/cvt225.
Wakui H, Tamura K, Masuda S, Tsurumi-Ikeya Y, Fujita M, Maeda A, et al. Enhanced angiotensin receptor-associated protein in renal tubule suppresses angiotensin-dependent hypertension. Hypertension. 2013;61(6):1203–10. https://doi.org/10.1161/HYPERTENSIONAHA.111.00572.
Azushima K, Ohki K, Wakui H, Uneda K, Haku S, Kobayashi R, et al. Adipocyte-specific enhancement of angiotensin II type 1 receptor-associated protein ameliorates diet-induced visceral obesity and insulin resistance. J Am Heart Assoc. 2017;6(3). https://doi.org/10.1161/JAHA.116.004488.
Uneda K, Wakui H, Maeda A, Azushima K, Kobayashi R, Haku S, et al. Angiotensin II type 1 receptor-associated protein regulates kidney aging and lifespan independent of angiotensin. J Am Heart Assoc. 2017;6(8). https://doi.org/10.1161/JAHA.117.006120.
Ohki K, Wakui H, Kishio N, Azushima K, Uneda K, Haku S, et al. Angiotensin II type 1 receptor-associated protein inhibits angiotensin II-induced insulin resistance with suppression of oxidative stress in skeletal muscle tissue. Sci Rep. 2018;8(1):2846. https://doi.org/10.1038/s41598-018-21270-8.
Ohsawa M, Tamura K, Wakui H, Maeda A, Dejima T, Kanaoka T, et al. Deletion of the angiotensin II type 1 receptor-associated protein enhances renal sodium reabsorption and exacerbates angiotensin II-mediated hypertension. Kidney Int. 2014;86(3):570–81. https://doi.org/10.1038/ki.2014.95.
Maeda A, Tamura K, Wakui H, Dejima T, Ohsawa M, Azushima K, et al. Angiotensin receptor-binding protein ATRAP/Agtrap inhibits metabolic dysfunction with visceral obesity. J Am Heart Assoc. 2013;2(4):e000312. https://doi.org/10.1161/JAHA.113.000312.
Guo DF, Chenier I, Tardif V, Orlov SN, Inagami T. Type 1 angiotensin II receptor-associated protein ARAP1 binds and recycles the receptor to the plasma membrane. Biochem Biophys Res Commun. 2003;310(4):1254–65. https://doi.org/10.1016/j.bbrc.2003.09.154.
•• Horiuchi M, Iwanami J, Mogi M. Regulation of angiotensin II receptors beyond the classical pathway. Clin Sci. 2012;123(4):193–203. https://doi.org/10.1042/CS20110677This paper summarizes mechanisms of angiotensin II receptors-associated proteins that regulate the function of angiotensin II type 1 and type 2 receptors.
Guo DF, Chenier I, Lavoie JL, Chan JS, Hamet P, Tremblay J, et al. Development of hypertension and kidney hypertrophy in transgenic mice overexpressing ARAP1 gene in the kidney. Hypertension. 2006;48(3):453–9. https://doi.org/10.1161/01.HYP.0000230664.32874.52.
Mederle K, Schweda F, Kattler V, Doblinger E, Miyata K, Hocherl K, et al. The angiotensin II AT1 receptor-associated protein Arap1 is involved in sepsis-induced hypotension. Crit Care. 2013;17(4):R130. https://doi.org/10.1186/cc12809.
Doblinger E, Hocherl K, Mederle K, Kattler V, Walter S, Hansen PB, et al. Angiotensin AT1 receptor-associated protein Arap1 in the kidney vasculature is suppressed by angiotensin II. Am J Physiol Renal Physiol. 2012;302(10):F1313–24. https://doi.org/10.1152/ajprenal.00620.2011.
Cook JL, Re RN, de Haro DL, Abadie JM, Peters M, Alam J. The trafficking protein GABARAP binds to and enhances plasma membrane expression and function of the angiotensin II type 1 receptor. Circ Res. 2008;102(12):1539–47. https://doi.org/10.1161/CIRCRESAHA.108.176594.
Zhang X, Wang H, Duvernay MT, Zhu S, Wu G. The angiotensin II type 1 receptor C-terminal Lys residues interact with tubulin and modulate receptor export trafficking. PLoS One. 2013;8(2):e57805. https://doi.org/10.1371/journal.pone.0057805.
Zhu S, Zhang M, Davis JE, Wu WH, Surrao K, Wang H, et al. A single mutation in helix 8 enhances the angiotensin II type 1a receptor transport and signaling. Cell Signal. 2015;27(12):2371–9. https://doi.org/10.1016/j.cellsig.2015.08.020.
Siragy HM, Carey RM. The subtype-2 (AT2) angiotensin receptor regulates renal cyclic guanosine 3′, 5′-monophosphate and AT1 receptor-mediated prostaglandin E2 production in conscious rats. J Clin Invest. 1996;97(8):1978–82. https://doi.org/10.1172/JCI118630.
Siragy HM, Jaffa AA, Margolius HS, Carey RM. Renin-angiotensin system modulates renal bradykinin production. Am J Phys. 1996;271(4 Pt 2):R1090–5. https://doi.org/10.1152/ajpregu.1996.271.4.R1090.
Siragy HM, Carey RM. The subtype 2 (AT2) angiotensin receptor mediates renal production of nitric oxide in conscious rats. J Clin Invest. 1997;100(2):264–9. https://doi.org/10.1172/JCI119531.
Siragy HM, Jaffa AA, Margolius HS. Bradykinin B2 receptor modulates renal prostaglandin E2 and nitric oxide. Hypertension. 1997;29(3):757–62.
Tsutsumi Y, Matsubara H, Masaki H, Kurihara H, Murasawa S, Takai S, et al. Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. J Clin Invest. 1999;104(7):925–35. https://doi.org/10.1172/JCI7886.
Abadir PM, Carey RM, Siragy HM. Angiotensin AT2 receptors directly stimulate renal nitric oxide in bradykinin B2-receptor-null mice. Hypertension. 2003;42(4):600–4. https://doi.org/10.1161/01.HYP.0000090323.58122.5C.
You D, Loufrani L, Baron C, Levy BI, Widdop RE, Henrion D. High blood pressure reduction reverses angiotensin II type 2 receptor-mediated vasoconstriction into vasodilation in spontaneously hypertensive rats. Circulation. 2005;111(8):1006–11. https://doi.org/10.1161/01.CIR.0000156503.62815.48.
Moltzer E, Verkuil AV, van Veghel R, Danser AH, van Esch JH. Effects of angiotensin metabolites in the coronary vascular bed of the spontaneously hypertensive rat: loss of angiotensin II type 2 receptor-mediated vasodilation. Hypertension. 2010;55(2):516–22. https://doi.org/10.1161/HYPERTENSIONAHA.109.145037.
Kurisu S, Ozono R, Oshima T, Kambe M, Ishida T, Sugino H, et al. Cardiac angiotensin II type 2 receptor activates the kinin/NO system and inhibits fibrosis. Hypertension. 2003;41(1):99–107. https://doi.org/10.1161/01.hyp.0000050101.90932.14.
Yan X, Price RL, Nakayama M, Ito K, Schuldt AJ, Manning WJ, et al. Ventricular-specific expression of angiotensin II type 2 receptors causes dilated cardiomyopathy and heart failure in transgenic mice. Am J Physiol Heart Circ Physiol. 2003;285(5):H2179–87. https://doi.org/10.1152/ajpheart.00361.2003.
• Xu J, Sun Y, Carretero OA, Zhu L, Harding P, Shesely EG, et al. Effects of cardiac overexpression of the angiotensin II type 2 receptor on remodeling and dysfunction in mice post-myocardial infarction. Hypertension. 2014;63(6):1251–9. https://doi.org/10.1161/HYPERTENSIONAHA.114.03247This paper demonstrates that the beneficial or detrimental effects of angiotensin II type 2 receptor largely depend on the degree of its expression.
Knowle D, Ahmed S, Pulakat L. Identification of an interaction between the angiotensin II receptor sub-type AT2 and the ErbB3 receptor, a member of the epidermal growth factor receptor family. Regul Pept. 2000;87(1–3):73–82.
Senbonmatsu T, Saito T, Landon EJ, Watanabe O, Price E Jr, Roberts RL, et al. A novel angiotensin II type 2 receptor signaling pathway: possible role in cardiac hypertrophy. EMBO J. 2003;22(24):6471–82. https://doi.org/10.1093/emboj/cdg637.
Fritz RD, Radziwill G. The scaffold protein CNK1 interacts with the angiotensin II type 2 receptor. Biochem Biophys Res Commun. 2005;338(4):1906–12. https://doi.org/10.1016/j.bbrc.2005.10.168.
Pulakat L, Cooper S, Knowle D, Mandavia C, Bruhl S, Hetrick M, et al. Ligand-dependent complex formation between the angiotensin II receptor subtype AT2 and Na+/H+ exchanger NHE6 in mammalian cells. Peptides. 2005;26(5):863–73. https://doi.org/10.1016/j.peptides.2004.12.015.
Kang KH, Park SY, Rho SB, Lee JH. Tissue inhibitor of metalloproteinases-3 interacts with angiotensin II type 2 receptor and additively inhibits angiogenesis. Cardiovasc Res. 2008;79(1):150–60. https://doi.org/10.1093/cvr/cvn072.
Nouet S, Amzallag N, Li JM, Louis S, Seitz I, Cui TX, et al. Trans-inactivation of receptor tyrosine kinases by novel angiotensin II AT2 receptor-interacting protein, ATIP. J Biol Chem. 2004;279(28):28989–97. https://doi.org/10.1074/jbc.M403880200.
Wruck CJ, Funke-Kaiser H, Pufe T, Kusserow H, Menk M, Schefe JH, et al. Regulation of transport of the angiotensin AT2 receptor by a novel membrane-associated Golgi protein. Arterioscler Thromb Vasc Biol. 2005;25(1):57–64. https://doi.org/10.1161/01.ATV.0000150662.51436.14.
Fujita T, Mogi M, Min LJ, Iwanami J, Tsukuda K, Sakata A, et al. Attenuation of cuff-induced neointimal formation by overexpression of angiotensin II type 2 receptor-interacting protein 1. Hypertension. 2009;53(4):688–93. https://doi.org/10.1161/HYPERTENSIONAHA.108.128140.
Min LJ, Mogi M, Iwanami J, Jing F, Tsukuda K, Ohshima K, et al. Angiotensin II type 2 receptor-interacting protein prevents vascular senescence. J Am Soc Hypertens. 2012;6(3):179–84. https://doi.org/10.1016/j.jash.2012.01.006.
Jing F, Mogi M, Min LJ, Ohshima K, Nakaoka H, Tsukuda K, et al. Effect of angiotensin II type 2 receptor-interacting protein on adipose tissue function via modulation of macrophage polarization. PLoS One. 2013;8(4):e60067. https://doi.org/10.1371/journal.pone.0060067.
Ito S, Asakura M, Liao Y, Min KD, Takahashi A, Shindo K, et al. Identification of the Mtus1 splice variant as a novel inhibitory factor against cardiac hypertrophy. J Am Heart Assoc. 2016;5(7). https://doi.org/10.1161/JAHA.116.003521.
Yaffe MP, Schatz G. Two nuclear mutations that block mitochondrial protein import in yeast. Proc Natl Acad Sci U S A. 1984;81(15):4819–23. https://doi.org/10.1073/pnas.81.15.4819.
• Santos RA, Simoes E, Silva AC, Maric C, Silva DM, Machado RP, et al. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci U S A. 2003;100(14):8258–63. https://doi.org/10.1073/pnas.1432869100This is one of the first papers describing that Mas receptor is a receptor for angotensin (1–7) to exert anti-diuretic actions and arterial relaxation response.
Passos-Silva DG, Verano-Braga T, Santos RA. Angiotensin-(1-7): beyond the cardio-renal actions. Clin Sci. 2013;124(7):443–56. https://doi.org/10.1042/CS20120461.
Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM. Angiotensin-(1-7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension. 2007;49(1):185–92. https://doi.org/10.1161/01.HYP.0000251865.35728.2f.
Dias-Peixoto MF, Santos RA, Gomes ER, Alves MN, Almeida PW, Greco L, et al. Molecular mechanisms involved in the angiotensin-(1-7)/Mas signaling pathway in cardiomyocytes. Hypertension. 2008;52(3):542–8. https://doi.org/10.1161/HYPERTENSIONAHA.108.114280.
Karnik SS, Singh KD, Tirupula K, Unal H. Significance of angiotensin 1-7 coupling with MAS1 receptor and other GPCRs to the renin-angiotensin system: IUPHAR Review 22. Br J Pharmacol. 2017;174(9):737–53. https://doi.org/10.1111/bph.13742.
Shimada K, Furukawa H, Wada K, Wei Y, Tada Y, Kuwabara A, et al. Angiotensin-(1-7) protects against the development of aneurysmal subarachnoid hemorrhage in mice. J Cereb Blood Flow Metab. 2015;35(7):1163–8. https://doi.org/10.1038/jcbfm.2015.30.
Ohshima K, Mogi M, Nakaoka H, Iwanami J, Min LJ, Kanno H, et al. Possible role of angiotensin-converting enzyme 2 and activation of angiotensin II type 2 receptor by angiotensin-(1-7) in improvement of vascular remodeling by angiotensin II type 1 receptor blockade. Hypertension. 2014;63(3):e53–9. https://doi.org/10.1161/HYPERTENSIONAHA.113.02426.
Villela DC, Passos-Silva DG, Santos RA. Alamandine: a new member of the angiotensin family. Curr Opin Nephrol Hypertens. 2014;23(2):130–4. https://doi.org/10.1097/01.mnh.0000441052.44406.92.
• Lautner RQ, Villela DC, Fraga-Silva RA, Silva N, Verano-Braga T, Costa-Fraga F, et al. Discovery and characterization of alamandine: a novel component of the renin-angiotensin system. Circ Res. 2013;112(8):1104–11. https://doi.org/10.1161/CIRCRESAHA.113.301077This is the first paper that discovers and characterizes a novel component of the renin-angiotensin system, alamandine.
Jesus ICG, Scalzo S, Alves F, Marques K, Rocha-Resende C, Bader M, et al. Alamandine acts via MrgD to induce AMPK/NO activation against ANG II hypertrophy in cardiomyocytes. Am J Physiol Cell Physiol. 2018;314(6):C702–C11. https://doi.org/10.1152/ajpcell.00153.2017.
Tetzner A, Gebolys K, Meinert C, Klein S, Uhlich A, Trebicka J, et al. G-protein-coupled receptor MrgD is a receptor for angiotensin-(1-7) involving adenylyl cyclase, cAMP, and phosphokinase a. Hypertension. 2016;68(1):185–94. https://doi.org/10.1161/HYPERTENSIONAHA.116.07572.
Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240(4854):889–95. https://doi.org/10.1126/science.3283939.
Stockand JD. New ideas about aldosterone signaling in epithelia. Am J Physiol Renal Physiol. 2002;282(4):F559–76. https://doi.org/10.1152/ajprenal.00320.2001.
Rafiq K, Hitomi H, Nakano D, Nishiyama A. Pathophysiological roles of aldosterone and mineralocorticoid receptor in the kidney. J Pharmacol Sci. 2011;115(1):1–7.
Nishiyama A, Kobori H. Independent regulation of renin-angiotensin-aldosterone system in the kidney. Clin Exp Nephrol. 2018;22(6):1231–9. https://doi.org/10.1007/s10157-018-1567-1.
Shibata H, Itoh H. Mineralocorticoid receptor-associated hypertension and its organ damage: clinical relevance for resistant hypertension. Am J Hypertens. 2012;25(5):514–23. https://doi.org/10.1038/ajh.2011.245.
Funder JW, Pearce PT, Smith R, Smith AI. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science. 1988;242(4878):583–5. https://doi.org/10.1126/science.2845584.
Baker ME, Funder JW, Kattoula SR. Evolution of hormone selectivity in glucocorticoid and mineralocorticoid receptors. J Steroid Biochem Mol Biol. 2013;137:57–70. https://doi.org/10.1016/j.jsbmb.2013.07.009.
Rafiq K, Nakano D, Ihara G, Hitomi H, Fujisawa Y, Ohashi N, et al. Effects of mineralocorticoid receptor blockade on glucocorticoid-induced renal injury in adrenalectomized rats. J Hypertens. 2011;29(2):290–8.
Vitellius G, Trabado S, Bouligand J, Delemer B, Lombes M. Pathophysiology of glucocorticoid signaling. Ann Endocrinol. 2018;79(3):98–106. https://doi.org/10.1016/j.ando.2018.03.001.
•• Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, et al. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med. 2008;14(12):1370–6. https://doi.org/10.1038/nm.1879This paper reveals the aldosterone-independent activation of MR and that the Rac 1-mediated MR activation leads to the kidney damages, suggesting the importance of the therapeutic MR target in the organ-protective effects.
Shibata S, Ishizawa K, Uchida S. Mineralocorticoid receptor as a therapeutic target in chronic kidney disease and hypertension. Hypertens Res. 2017;40(3):221–5. https://doi.org/10.1038/hr.2016.137.
Uddin S, Lekmine F, Sharma N, Majchrzak B, Mayer I, Young PR, et al. The Rac1/p38 mitogen-activated protein kinase pathway is required for interferon alpha-dependent transcriptional activation but not serine phosphorylation of Stat proteins. J Biol Chem. 2000;275(36):27634–40. https://doi.org/10.1074/jbc.M003170200.
Aikawa R, Komuro I, Yamazaki T, Zou Y, Kudoh S, Zhu W, et al. Rho family small G proteins play critical roles in mechanical stress-induced hypertrophic responses in cardiac myocytes. Circ Res. 1999;84(4):458–66. https://doi.org/10.1161/01.res.84.4.458.
Shibata S, Mu S, Kawarazaki H, Muraoka K, Ishizawa K, Yoshida S, et al. Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway. J Clin Invest. 2011;121(8):3233–43. https://doi.org/10.1172/JCI43124.
Nagase M, Ayuzawa N, Kawarazaki W, Ishizawa K, Ueda K, Yoshida S, et al. Oxidative stress causes mineralocorticoid receptor activation in rat cardiomyocytes: role of small GTPase Rac1. Hypertension. 2012;59(2):500–6. https://doi.org/10.1161/HYPERTENSIONAHA.111.185520.
Ayuzawa N, Nagase M, Ueda K, Nishimoto M, Kawarazaki W, Marumo T, et al. Rac1-mediated activation of mineralocorticoid receptor in pressure overload-induced cardiac injury. Hypertension. 2016;67(1):99–106. https://doi.org/10.1161/HYPERTENSIONAHA.115.06054.
• Ito S, Itoh H, Rakugi H, Okuda Y, Yamakawa S. Efficacy and safety of esaxerenone (CS-3150) for the treatment of essential hypertension: a phase 2 randomized, placebo-controlled, double-blind study. J Hum Hypertens. 2019;33(7):542–51. https://doi.org/10.1038/s41371-019-0207-xThis paper demonstrates that esaxerenone reduces the BP dose-dependently and does not cause sex-hormone-related adverse events.
Arai K, Homma T, Morikawa Y, Ubukata N, Tsuruoka H, Aoki K, et al. Pharmacological profile of CS-3150, a novel, highly potent and selective non-steroidal mineralocorticoid receptor antagonist. Eur J Pharmacol. 2015;761:226–34. https://doi.org/10.1016/j.ejphar.2015.06.015.
Arai K, Tsuruoka H, Homma T. CS-3150, a novel non-steroidal mineralocorticoid receptor antagonist, prevents hypertension and cardiorenal injury in Dahl salt-sensitive hypertensive rats. Eur J Pharmacol. 2015;769:266–73. https://doi.org/10.1016/j.ejphar.2015.11.028.
Arai K, Morikawa Y, Ubukata N, Tsuruoka H, Homma T. CS-3150, a novel nonsteroidal mineralocorticoid receptor antagonist, shows preventive and therapeutic effects on renal injury in Deoxycorticosterone acetate/salt-induced hypertensive rats. J Pharmacol Exp Ther. 2016;358(3):548–57. https://doi.org/10.1124/jpet.116.234765.
Bhuiyan AS, Rafiq K, Kobara H, Masaki T, Nakano D, Nishiyama A. Effect of a novel nonsteroidal selective mineralocorticoid receptor antagonist, esaxerenone (CS-3150), on blood pressure and renal injury in high salt-treated type 2 diabetic mice. Hypertens Res. 2019;42(6):892–902. https://doi.org/10.1038/s41440-019-0211-0.
Li L, Guan Y, Kobori H, Morishita A, Kobara H, Masaki T, et al. Effects of the novel nonsteroidal mineralocorticoid receptor blocker, esaxerenone (CS-3150), on blood pressure and urinary angiotensinogen in low-renin Dahl salt-sensitive hypertensive rats. Hypertens Res. 2019;42(6):769–78. https://doi.org/10.1038/s41440-018-0187-1.
Ito S, Shikata K, Nangaku M, Okuda Y, Sawanobori T. Efficacy and safety of esaxerenone (CS-3150) for the treatment of type 2 diabetes with microalbuminuria: a randomized, double-blind, placebo-controlled, phase II trial. Clin J Am Soc Nephrol. 2019. https://doi.org/10.2215/CJN.14751218.
Itoh H, Ito S, Rakugi H, Okuda Y, Nishioka S. Efficacy and safety of dosage-escalation of low-dosage esaxerenone added to a RAS inhibitor in hypertensive patients with type 2 diabetes and albuminuria: a single-arm, open-label study. Hypertens Res. 2019;42:1572–81. https://doi.org/10.1038/s41440-019-0270-2.
Kolkhof P, Delbeck M, Kretschmer A, Steinke W, Hartmann E, Barfacker L, et al. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J Cardiovasc Pharmacol. 2014;64(1):69–78. https://doi.org/10.1097/FJC.0000000000000091.
Grune J, Benz V, Brix S, Salatzki J, Blumrich A, Hoft B, et al. Steroidal and nonsteroidal mineralocorticoid receptor antagonists cause differential cardiac gene expression in pressure overload-induced cardiac hypertrophy. J Cardiovasc Pharmacol. 2016;67(5):402–11. https://doi.org/10.1097/FJC.0000000000000366.
Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. Jama. 2015;314(9):884–94. https://doi.org/10.1001/jama.2015.10081.
Katayama S, Yamada D, Nakayama M, Yamada T, Myoishi M, Kato M, et al. A randomized controlled study of finerenone versus placebo in Japanese patients with type 2 diabetes mellitus and diabetic nephropathy. J Diabetes Complicat. 2017;31(4):758–65. https://doi.org/10.1016/j.jdiacomp.2016.11.021.
Pitt B, Kober L, Ponikowski P, Gheorghiade M, Filippatos G, Krum H, et al. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J. 2013;34(31):2453–63. https://doi.org/10.1093/eurheartj/eht187.
Filippatos G, Anker SD, Bohm M, Gheorghiade M, Kober L, Krum H, et al. A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur Heart J. 2016;37(27):2105–14. https://doi.org/10.1093/eurheartj/ehw132.
Sato N, Ajioka M, Yamada T, Kato M, Myoishi M, Yamada T, et al. A randomized controlled study of finerenone vs. eplerenone in Japanese patients with worsening chronic heart failure and diabetes and/or chronic kidney disease. Circ J. 2016;80(5):1113–22. https://doi.org/10.1253/circj.CJ-16-0122.
Acknowledgments
We thank Mitchell Arico from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Prevention of Hypertension: Public Health Challenges
Rights and permissions
About this article
Cite this article
Azushima, K., Morisawa, N., Tamura, K. et al. Recent Research Advances in Renin-Angiotensin-Aldosterone System Receptors. Curr Hypertens Rep 22, 22 (2020). https://doi.org/10.1007/s11906-020-1028-6
Published:
DOI: https://doi.org/10.1007/s11906-020-1028-6