Abstract
Purpose of Review
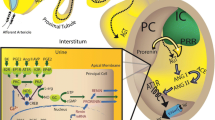
In this review, we summarized the current knowledge of connecting tubule-glomerular feedback (CTGF), a novel mechanism of renal microcirculation regulation that integrates sodium handling in the connecting tubule (CNT) with kidney hemodynamics.
Recent Findings
Connecting tubule-glomerular feedback is a crosstalk communication between the CNT and the afferent arteriole (Af-Art), initiated by sodium chloride through the epithelial sodium channel (ENaC). High sodium in the CNT induces Af-Art vasodilation, increasing glomerular pressure and the glomerular filtration rate and favoring sodium excretion. CTGF antagonized and reset tubuloglomerular feedback and thus increased sodium excretion. CTGF is absent in spontaneous hypertensive rats and is overactivated in Dahl salt-sensitive rats. CTGF is also modulated by angiotensin II and aldosterone.
Summary
CTGF is a feedback mechanism that integrates sodium handling in the CNT with glomerular hemodynamics. Lack of CTGF could promote hypertension, and CTGF overactivation may favor glomerular damage and proteinuria. More studies are needed to explore the alterations in renal microcirculation and the role of these alterations in the genesis of hypertension and glomerular damage in animals and humans.
Key Points
• CTGF is a vasodilator mechanism that regulates afferent arteriole resistance.
• CTGF is absent in spontaneous hypertensive rats and overactivated in Dahl salt-sensitive rats.
• CTGF in excess may promote glomerular damage and proteinuria, while the absence may participate in sodium retention and hypertension.




Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Guyton AC. Blood pressure control—special role of the kidneys and body fluids. Science (New York, NY). 1991;252(5014):1813–6.
Frame AA, Wainford RD. Renal sodium handling and sodium sensitivity. Kidney Res Clin Pract. 2017;36(2):117–31.
Neal CR, Arkill K, Bell JS, Betteridge KB, Bates DO, Winlove CP, et al. Novel haemodynamic structures in the human glomerulus. Am J Physiol Ren Physiol. 2018;315:F1370–84. https://doi.org/10.1152/ajprenal.00566.2017.
Brenner BM, Troy JL, Daugharty TM. The dynamics of glomerular ultrafiltration in the rat. J Clin Investig. 1971;50:1776–80.
Deen WM, Robertson CR, Brenner BM. Glomerular ultrafiltration. FedProc. 1974;33:14–20.
Brenner BM, Troy JL, Daugharty TM, Deen WM, Robertson CR. Dynamics of glomerular ultrafiltration in the rat. II. Plasma-flow dependence of GFR. Am J Physiol. 1972;223:1184–90.
•• Carlstrom M, Wilcox CS, Arendshorst WJ. Renal autoregulation in health and disease. Physiol Rev. 2015;95(2):405–511 This is an extensive review that describe the renal autoregulation mechanisms with very precised details.
• Ren Y, Garvin JL, Liu R, Carretero OA. Crosstalk between the connecting tubule and the afferent arteriole regulates renal microcirculation. Kidney Int. 2007;71(11):1116–21 This is the first description of CTGF.
Peti-Peterdi J, Bebok Z, Lapointe JY, Bell PD. Novel regulation of cell [Na(+)] in macula densa cells: apical Na(+) recycling by H-K-ATPase. Am J Physiol Ren Physiol. 2002;282(2):F324–9.
Komlosi P, Peti-Peterdi J, Fuson AL, Fintha A, Rosivall L, Bell PD. Macula densa basolateral ATP release is regulated by luminal [NaCl] and dietary salt intake. Am J Physiol Ren Physiol. 2004;286:F1054–F8.
Ren Y, Garvin JL, Liu R, Carretero OA. Role of macula densa adenosine triphosphate (ATP) in tubuloglomerular feedback. Kidney Int. 2004;66(4):1479–85.
Kirk KL, Bell PD, Barfuss DW, Ribadeneira M. Direct visualization of the isolated and perfused macula densa. Am J Physiol. 1985;248:F890–F4.
Barajas L, Powers K, Carretero OA, Scicli AG, Inagami T. Immunocytochemical localization of renin and kallikrein in the rat renal cortex. Kidney Int. 1986;29(5):965–70.
Dorup J, Morsing P, Rasch R. Tubule-tubule and tubule-arteriole contacts in rat kidney distal nephrons. A morphologic study based on computer-assisted three-dimensional reconstructions. Lab Investig. 1992;67(6):761–9.
Loffing J, Korbmacher C. Regulated sodium transport in the renal connecting tubule (CNT) via the epithelial sodium channel (ENaC). Pflugers Arch Eur J Physiol. 2009;458(1):111–35.
Frindt G, Palmer LG. Na channels in the rat connecting tubule. Am J Physiol Ren Physiol. 2004;286(4):F669–74.
Wall SM, Lazo-Fernandez Y. The role of pendrin in renal physiology. Annu Rev Physiol. 2015;77:363–78.
Jacques T, Picard N, Miller RL, Riemondy KA, Houillier P, Sohet F, et al. Overexpression of pendrin in intercalated cells produces chloride-sensitive hypertension. J Am Soc Nephrol. 2013;24(7):1104–13.
Wall SM. Renal intercalated cells and blood pressure regulation. Kidney Res Clin Pract. 2017;36(4):305–17.
• Liu R, Layton AT. Modeling the effects of positive and negative feedback in kidney blood flow control. Math Biosci. 2016;276:8–18 This study explore the effects of CTGF on TGF.
Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, et al. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci U S A. 2001;98:9983–8.
Ren Y, D’Ambrosio MA, Garvin JL, Wang H, Carretero OA. Possible mediators of connecting tubule glomerular feedback. Hypertension. 2009;53(part 2):319–23.
Ren Y, D’Ambrosio MA, Wang H, Garvin JL, Carretero OA. Participation of prostaglandin E 2 and EP4 receptors in connecting tubule glomerular feedback (CTGF) [abstract]. Hypertension. 2012;60(3 Supplement):A33.
Ren Y, D’Ambrosio MA, Wang H, Peterson EL, Garvin JL, Carretero OA. Mechanisms of angiotensin II-enhanced connecting tubule glomerular feedback. Am J Physiol Ren Physiol. 2012;303(2):F259–F65.
Ren Y, D’Ambrosio MA, Garvin JL, Leung P, Kutskill K, Wang H, et al. Aldosterone sensitizes connecting tubule glomerular feedback via the aldosterone receptor GPR30. Am J Physiol Ren Physiol. 2014;307(4):F427–34.
Romero CA, Peixoto AJ, Orias M. Estimated GFR or albuminuria: which one is really associated with resistant hypertension? Semin Nephrol. 2014;34(5):492–7.
Brown R, Ollerstam A, Persson AE. Neuronal nitric oxide synthase inhibition sensitizes the tubuloglomerular feedback mechanism after volume expansion. Kidney Int. 2004;65(4):1349–56.
Wang H, D’Ambrosio MA, Garvin JL, Ren Y, Carretero OA. Connecting tubule glomerular feedback in hypertension. Hypertension. 2013;62(4):738–45.
Monu SR, Ren Y, Masjoan-Juncos JX, Kutskill K, Wang H, Kumar N, et al. Connecting tubule glomerular feedback mediates tubuloglomerular feedback resetting after unilateral nephrectomy. Am J Physiol Ren Physiol. 2018;315(4):F806–F11.
Frohlich ED, Messerli FH, Dunn FG, Oigman W, Ventura HO, Sundgaard-Riise K. Greater renal vascular involvement in the black patient with essential hypertension. A comparison of systemic and renal hemodynamics in black and white patients. Miner Electrolyte Metab. 1984;10(3):173–7.
Weir MR. Salt intake and hypertensive renal injury in African-Americans. A therapeutic perspective. Am J Hypertens. 1995;8(6):635–44.
Raij L, Azar S, Keane WF. Role of hypertension in progressive glomerular immune injury. Hypertension. 1985;7(3 Pt 1):398–404.
Pavlov TS, Staruschenko A. Involvement of ENaC in the development of salt-sensitive hypertension. Am J Physiol Ren Physiol. 2017;313(2):F135–F40.
Wang H, Romero CA, Masjoan Juncos JX, Monu SR, Peterson EL, Carretero OA. Effect of salt intake on afferent arteriolar dilatation: role of connecting tubule glomerular feedback (CTGF). Am J Physiol Ren Physiol. 2017;313(6):F1209–F15.
Holstein-Rathlou NH, Leyssac PP. TGF-mediated oscillations in the proximal intratubular pressure: differences between spontaneously hypertensive rats and Wistar-Kyoto rats. Acta Physiol Scand. 1986;126(3):333–9.
Bianchi G, Fox U, Di Francesco GF, Giovanetti AM, Pagetti D. Blood pressure changes produced by kidney cross-transplantation between spontaneously hypertensive rats and normotensive rats. Clin Sci Mol Med. 1974;47(5):435–48.
Song J, Wang L, Fan F, Wei J, Zhang J, Lu Y, et al. Role of the primary cilia on the macula Densa and thick ascending limbs in regulation of sodium excretion and hemodynamics. Hypertension. 2017;70(2):324–33.
Lu Y, Wei J, Stec DE, Roman RJ, Ge Y, Cheng L, et al. Macula Densa nitric oxide synthase 1beta protects against salt-sensitive hypertension. J Am Soc Nephrol. 2016;27(8):2346–56.
Romero CA, Monu S, Knight R, Carretero OA, editors. Connecting tubule-glomerular feedback (CTGF) in renal hemodynamics and blood pressure (BP) after unilateral nephrectomy (UNX). Hypertension; 2016: Vol 68, Issue Suppl_1 (Abstract P148), USA.
Stevens PE, Levin A. Kidney disease: improving global outcomes chronic kidney disease guideline development work group M. evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–30.
Kambham N, Markowitz GS, Valeri AM, Lin J, D’Agati VD. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59(4):1498–509.
Praga M, Morales E. The fatty kidney: obesity and renal disease. Nephron. 2017;136(4):273–6.
Maheshwari M, Romero CA, Monu SR, Kumar N, Liao TD, Peterson EL, et al. Renal protective effects of N-acetyl-Seryl-aspartyl-Lysyl-proline (ac-SDKP) in obese rats on a high-salt diet. Am J Hypertens. 2018;31(8):902–9.
Chagnac A, Herman M, Zingerman B, Erman A, Rozen-Zvi B, Hirsh J, et al. Obesity-induced glomerular hyperfiltration: its involvement in the pathogenesis of tubular sodium reabsorption. Nephrol Dial Transplant. 2008;23(12):3946–52.
Monu SR, Maheshwari M, Peterson EL, Carretero OA. Role of connecting tubule glomerular feedback in obesity related renal damage. Am J Physiol Ren Physiol. 2018. https://doi.org/10.1152/ajprenal.00227.2018.
Andersen H, Hansen PB, Bistrup C, Nielsen F, Henriksen JE, Jensen BL. Significant natriuretic and antihypertensive action of the epithelial sodium channel blocker amiloride in diabetic patients with and without nephropathy. J Hypertens. 2016;34(8):1621–9.
Williams B, MacDonald TM, Morant SV, Webb DJ, Sever P, McInnes GT, et al. Endocrine and haemodynamic changes in resistant hypertension, and blood pressure responses to spironolactone or amiloride: the PATHWAY-2 mechanisms substudies. Lancet Diabetes Endocrinol. 2018;6(6):464–75.
Viera AJ, Wouk N. Potassium disorders: hypokalemia and hyperkalemia. Am Fam Phys. 2015;92(6).
Sepehrdad R, Chander PN, Oruene A, Rosenfeld L, Levine S, Stier CT Jr. Amiloride reduces stroke and renalinjury in stroke-prone hypertensive rats. Am J Hypertens. 2003;16(4):312–8.
Zhang B, Xie S, Shi W, Yang Y. Amiloride off-target effect inhibits podocyte urokinase receptor expression and reduces proteinuria. Nephrol Dial Transplant. 2012;27(5):1746–55.
Xu LB, Chi N, Shi W. Amiloride, a urokinase-type plasminogen activator receptor (uTPA) inhibitor, reduces proteinurea in podocytes. Genet Mol Res. 2015;14(3):9518–29.
Vassalli JD, Belin D. Amiloride selectively inhibits the urokinase-type plasminogen activator. FEBS Lett. 1987;214(1):187–91.
Svenningsen P, Andersen H, Nielsen LH, Jensen BL. Urinary serine proteases and activation of ENaC in kidney—implications for physiological renal salt handling and hypertensive disorders with albuminuria. Pflugers Arch Eur J Physiol. 2015;467(3):531–42.
Staehr M, Buhl KB, Andersen RF, Svenningsen P, Nielsen F, Hinrichs GR, et al. Aberrant glomerular filtration of urokinase-type plasminogen activator in nephrotic syndrome leads to amiloride-sensitive plasminogen activation in urine. Am J Physiol Ren Physiol. 2015;309(3):F235–41.
Trimarchi H, Forrester M, Lombi F, Pomeranz V, Rana MS, Karl A, et al. Amiloride as an alternate adjuvant antiproteinuric agent in Fabry disease: the potential roles of plasmin and uPAR. Case Rep Nephrol. 2014;2014:854521.
Buhl KB, Oxlund CS, Friis UG, Svenningsen P, Bistrup C, Jacobsen IA, et al. Plasmin in urine from patients with type 2 diabetes and treatment-resistant hypertension activates ENaC in vitro. J Hypertens. 2014;32(8):1672–7 discussion 7.
Oxlund CS, Buhl KB, Jacobsen IA, Hansen MR, Gram J, Henriksen JE, et al. Amiloride lowers blood pressure and attenuates urine plasminogen activation in patients with treatment-resistant hypertension. J Am Soc Hypertens. 2014;8(12):872–81.
Acknowledgments
We would like to thank Dr. Tengis Pavlov for the assistance in the figure preparation.
Funding
This study was funded by the Heart, Lung, and Blood Institute of the National Institutes of Health under award number HL-028982. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of theTopical Collection on Mechanisms of Hypertension and Target-Organ Damage
Rights and permissions
About this article
Cite this article
Romero, C.A., Carretero, O.A. A Novel Mechanism of Renal Microcirculation Regulation: Connecting Tubule-Glomerular Feedback. Curr Hypertens Rep 21, 8 (2019). https://doi.org/10.1007/s11906-019-0911-5
Published:
DOI: https://doi.org/10.1007/s11906-019-0911-5