Abstract
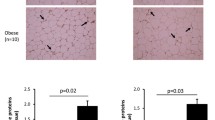
The chaperone protein heat shock protein (HSP) 70 has been shown to protect against obesity-associated insulin resistance. Induction of HSPs is thus considered an exciting therapeutic strategy for diabetes (DM). The aims of this study were to (1) determine HSP levels in plasma, hepatic, and pancreatic tissues of type 2 DM primates and (2) assess the relationship between chaperone proteins of the HSP family and cellular protection. We collected plasma from 24 type 2 DM and 25 normoglycemic control (CTL) cynomolgus macaques. A subset of DM monkeys had liver and pancreas samples available which were compared to a second group of CTL monkeys. We found that DM monkeys had 32% lower HSP70 in circulation which remained significant even after adjustment for the greater age and bodyweight of these monkeys (p < 0.001). The liver demonstrated a similar reductions in both HSP70 and 90 that was related to 50% lower levels of the transcription factor, heat shock factor 1 (HSF1; p = 0.03). Pancreatic tissue had the opposite expression pattern with significantly higher HSF1 (p = 0.004) and accordingly higher HSP70 and 90. Pancreas from DM monkeys had less nitrosative oxidation (p = 0.03) which was unaccounted for by superoxide dismutases and was negatively associated with HSP levels (r = −0.57, p = 0.009). HSF1/HSP deficiency exists in DM liver which may contribute to hepatic insulin resistance and this deficiency was reflected in lower circulating concentrations. Pancreas maintains HSP levels despite hyperglycemia, likely in an attempt to protect vulnerable beta cells from exocrine pancreatic damage and from stress associated with insulin hypersecretion.



Similar content being viewed by others
Abbreviations
- ADPN:
-
adiponectin
- BW:
-
bodyweight
- c-pep:
-
C-peptide
- CRP:
-
C-reactive protein
- CTL:
-
control
- DM:
-
diabetes
- DTT:
-
dithiothreitol
- EDTA:
-
ethylenediaminetetraacetic acid
- ER:
-
endoplasmic reticulum
- FBG:
-
fasting blood glucose
- IR:
-
insulin resistance
- HSF1:
-
heat shock factor 1
- HSP:
-
heat shock protein
- IL-6:
-
interleukin-6
- NADPH:
-
nicotinamide adenine dinucleotide phosphate
- ox-LDL:
-
oxidized ApoB lipoprotein
- SOD:
-
superoxide dismutase
- SEM:
-
standard error of the mean
References
American Diabetes Association (2007) Diagnosis and classification of diabetes mellitus. Diabetes Care 30(1):S42–S47
Aronson D (2003) Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens 21:3–12
Atalay M, Oksala NK, Laaksonen DE et al (2004) Exercise training modulates heat shock protein response in diabetic rats. J Appl Physiol 97:605–611
Bhagat L, Singh VP, Hietaranta AJ, Agrawal S, Steer ML, Saluja AK (2000) Heat shock protein 70 prevents secretagogue-induced cell injury in the pancreas by preventing intracellular trypsinogen activation. J Clin Invest 106:81–89
Bitar MS, Farook T, John B, Francis IM (1999) Heat-shock protein 72/73 and impaired wound healing in diabetic and hypercortisolemic states. Surgery 125:594–601
Bonora E, Targher G, Alberiche M et al (2000) Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 23:57–63
Bruce CR, Carey AL, Hawley JA, Febbraio MA (2003) Intramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes 52:2338–2345
Burkart V, Liu H, Bellmann K et al (2000) Natural resistance of human beta cells toward nitric oxide is mediated by heat shock protein 70. J Biol Chem 275:19521–19528
Chung J, Nguyen AK, Henstridge DC et al (2008) HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci U S A 105:1739–1744
Decker M, Hofflich H, Elias AN (2008) Thiazolidinediones and the preservation of beta-cell function, cellular proliferation and apoptosis. Diabetes Obes Metab 10(8):617–625
DeFronzo RA (2004) Pathogenesis of type 2 diabetes mellitus. Med Clin North Am 88:787–835 ix
Eizirik DL, Pipeleers DG, Ling Z, Welsh N, Hellerstrom C, Andersson A (1994) Major species differences between humans and rodents in the susceptibility to pancreatic beta-cell injury. Proc Natl Acad Sci U S A 91:9253–9256
Febbraio MA, Mesa JL, Chung J et al (2004) Glucose ingestion attenuates the exercise-induced increase in circulating heat shock protein 72 and heat shock protein 60 in humans. Cell Stress Chaperones 9:390–396
Feinstein DL, Galea E, Aquino DA, Li GC, Xu H, Reis DJ (1996) Heat shock protein 70 suppresses astroglial-inducible nitric-oxide synthase expression by decreasing NFkappaB activation. J Biol Chem 271:17724–17732
Gutsmann-Conrad A, Pahlavani MA, Heydari AR, Richardson A (1999) Expression of heat shock protein 70 decreases with age in hepatocytes and splenocytes from female rats. Mech Ageing Dev 107:255–270
Hooper PL (2007) Insulin signaling, GSK-3, heat shock proteins and the natural history of type 2 diabetes mellitus: a hypothesis. Metab Syndr Relat Disord 5:220–230
Horowitz M, Robinson SD (2007) Heat shock proteins and the heat shock response during hyperthermia and its modulation by altered physiological conditions. Prog Brain Res 162:433–446
Hotamisligil GS (2005) Role of endoplasmic reticulum stress and c-Jun NH2-terminal kinase pathways in inflammation and origin of obesity and diabetes. Diabetes 54(Suppl 2):S73–S78
Hsu AL, Murphy CT, Kenyon C (2003) Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 300:1142–1145
Jin X, Wang R, Xiao C et al (2004) Serum and lymphocyte levels of heat shock protein 70 in aging: a study in the normal Chinese population. Cell Stress Chaperones 9:69–75
Kurucz I, Morva A, Vaag A, Eriksson KF, Huang X, Groop L, Koranyi L (2002) Decreased expression of heat shock protein 72 in skeletal muscle of patients with type 2 diabetes correlates with insulin resistance. Diabetes 51:1102–1109
Laybutt DR, Kaneto H, Hasenkamp W et al (2002) Increased expression of antioxidant and antiapoptotic genes in islets that may contribute to beta-cell survival during chronic hyperglycemia. Diabetes 51:413–423
Laybutt DR, Glandt M, Xu G, Ahn YB, Trivedi N, Bonner-Weir S, Weir GC (2003) Critical reduction in beta-cell mass results in two distinct outcomes over time. Adaptation with impaired glucose tolerance or decompensated diabetes. J Biol Chem 278:2997–3005
Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ (2007) Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia 50:752–763
Linton S, Davies MJ, Dean RT (2001) Protein oxidation and ageing. Exp Gerontol 36:1503–1518
Lubec B, Hermon M, Hoeger H, Lubec G (1998) Aromatic hydroxylation in animal models of diabetes mellitus. FASEB J 12:1581–1587
Marini M, Lapalombella R, Canaider S et al (2004) Heat shock response by EBV-immortalized B-lymphocytes from centenarians and control subjects: a model to study the relevance of stress response in longevity. Exp Gerontol 39:83–90
McCarty MF (2006) Induction of heat shock proteins may combat insulin resistance. Med Hypotheses 66:527–534
McMurtry AL, Cho K, Young LJ, Nelson CF, Greenhalgh DG (1999) Expression of HSP70 in healing wounds of diabetic and nondiabetic mice. J Surg Res 86:36–41
Najemnikova E, Rodgers CD, Locke M (2007) Altered heat stress response following streptozotocin-induced diabetes. Cell Stress Chaperones 12:342–352
Ohkuwa T, Sato Y, Naoi M (1995) Hydroxyl radical formation in diabetic rats induced by streptozotocin. Life Sci 56:1789–1798
Okada M, Hasebe N, Aizawa Y, Izawa K, Kawabe J, Kikuchi K (2004) Thermal treatment attenuates neointimal thickening with enhanced expression of heat-shock protein 72 and suppression of oxidative stress. Circulation 109:1763–1768
Ooie T, Kajimoto M, Takahashi N et al (2005) Effects of insulin resistance on geranylgeranylacetone-induced expression of heat shock protein 72 and cardioprotection in high-fat diet rats. Life Sci 77:869–881
Ozcan U, Yilmaz E, Ozcan L et al (2006) Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313:1137–1140
Park J, Liu AY (2001) JNK phosphorylates the HSF1 transcriptional activation domain: role of JNK in the regulation of the heat shock response. J Cell Biochem 82:326–338
Patti ME, Butte AJ, Crunkhorn S et al (2003) Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A 100:8466–8471
Plum A, Agerso H, Andersen L (2000) Pharmacokinetics of the rapid-acting insulin analog, insulin aspart, in rats, dogs, and pigs, and pharmacodynamics of insulin aspart in pigs. Drug Metab Dispos 28:155–160
Rea IM, McNerlan S, Pockley AG (2001) Serum heat shock protein and anti-heat shock protein antibody levels in aging. Exp Gerontol 36:341–352
Ritzel RA, Butler AE, Rizza RA, Veldhuis JD, Butler PC (2006) Relationship between beta-cell mass and fasting blood glucose concentration in humans. Diabetes Care 29:717–718
Scheuner D, Vander Mierde D, Song B et al (2005) Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat Med 11:757–764
Swiecki C, Stojadinovic A, Anderson J, Zhao A, Dawson H, Shea-Donohue T (2003) Effect of hyperglycemia and nitric oxide synthase inhibition on heat tolerance and induction of heat shock protein 72 kDa in vivo. Am Surg 69:587–592
Turko IV, Murad F (2002) Protein nitration in cardiovascular diseases. Pharmacol Rev 54:619–634
Verbeke P, Clark BF, Rattan SI (2001) Reduced levels of oxidized and glycoxidized proteins in human fibroblasts exposed to repeated mild heat shock during serial passaging in vitro. Free Radic Biol Med 31:1593–1602
Wachlin G, Heine L, Kloting I, Dunger A, Hahn HJ, Schmidt S (2002) Stress response of pancreatic islets from diabetes prone BB rats of different age. Autoimmunity 35:389–395
Weber SM, Chambers KT, Bensch KG, Scarim AL, Corbett JA (2004) PPARgamma ligands induce ER stress in pancreatic beta-cells: ER stress activation results in attenuation of cytokine signaling. Am J Physiol Endocrinol Metab 287:E1171–E1177
Welsh N, Margulis B, Borg LA et al (1995) Differences in the expression of heat-shock proteins and antioxidant enzymes between human and rodent pancreatic islets: implications for the pathogenesis of insulin-dependent diabetes mellitus. Mol Med 1:806–820
Yamagishi N, Nakayama K, Wakatsuki T, Hatayama T (2001) Characteristic changes of stress protein expression in streptozotocin-induced diabetic rats. Life Sci 69:2603–2609
Acknowledgements
Funding for this study was provided by NIH Cardiovascular Pathology Training Grant 5T32HL07115 (KK), Wake Forest University School of Medicine, and Pfizer. The authors gratefully recognize Mr. Mickey Flynn for his editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kavanagh, K., Zhang, L. & Wagner, J.D. Tissue-specific regulation and expression of heat shock proteins in type 2 diabetic monkeys. Cell Stress and Chaperones 14, 291–299 (2009). https://doi.org/10.1007/s12192-008-0084-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-008-0084-7