Abstract
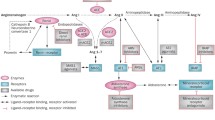
Interruption of the renin-angiotensin-aldosterone system (RAAS) at different levels is target-organ protective in several disease states; however, complete blockade is unlikely to be achieved due to escape mechanisms whenever blockade is attempted, incomplete knowledge of the role of all elements of the RAAS, and lack of pharmacotherapy against some elements that have been shown to contribute to disease states. Aldosterone has been overlooked as a mediator of RAAS escape and a key factor in target-organ injury despite the use of available RAAS blockers. Aldosterone is thought to play a role in the development of hypertension, alteration in vascular structure, vascular smooth muscle hypertrophy, endothelial dysfunction, structural renal injury, proteinuria, left ventricular remodeling, collagen synthesis, and myocardial fibrosis. Aldosterone receptor antagonists have been shown to antagonize all these effects in experimental models. Clinical trials with aldosterone antagonists showed an improvement in survival and left ventricular mass index in patients with congestive heart failure, and a reduction in urinary protein excretion and left ventricular mass index in patients with type 2 diabetes and early nephropathy who developed aldosterone synthesis escape. Consequently, aldosterone receptor antagonists may have specific benefits for reducing target-organ injury, particularly if there is evidence of RAAS escape.
Similar content being viewed by others
References and Recommended Reading
Basso N, Terragno NA: History about the discovery of the renin-angiotensin system. Hypertension 2001, 38:1246–1249.
Rohrwasser A, Morgan T, Dillon HF: Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension 1999, 34:1265–1274.
Bader M: Role of the local renin-angiotensin system in cardiac damage: a minireview focussing on transgenic animal models. J Mol Cell Cardiol 2002, 34:1455–1462.
Bader M, Ganten D: It’s renin in the brain: transgenic animals elucidate the brain renin angiotensin ystem. Circ Res 2002, 90:8–10.
Ganten D, Hermann K, Bayer C, et al.: Angiotensin synthesis in the brain and increased turnover in hypertensive rats. Science 1983, 221:869–871.
Senanayake PD, Moriguchi A, Kumagai H, et al.: Increased expression of angiotensin peptides in the brain of transgenic hypertensive rats. Peptides 1994, 15:919–926.
Shibata K, Komatsu C, Misumi Y, Furukawa T: Developmental differences of angiotensinogen mRNA in the preoptic area between spontaneously hypertensive and age-matched Wistar-Kyoto rats. Brain Res Mol Brain Res 1993, 19:115–120.
Wright JW, Harding JW: Regulatory role of brain angiotensins in the control of physiological and behavioral responses. Brain Res Brain Res Rev 1992, 17:227–262.
Unger T, Culman J, Gohlke P: Angiotensin II receptor blockade and end-organ protection: pharmacological rationale and evidence. J Hypertens Suppl 1998, 16:S3-S9.
Grekin RJ, Terris JM, Bohr DF: Electrolyte and hormonal effects of deoxycorticosterone acetate in young pigs. Hypertension 1980, 2:326–332.
Grekin RJ, Sider RS: Aldosterone receptor measurements during changes in dietary sodium. Endocrinology 1981, 108:109–112.
Lehoux JG, Bellabarba D, Beaudry C: Corticosteroid receptors in the kidney of chick embryo. III. Nature, properties, and ontogeny of aldosterone receptor. Gen Comp Endocrinol 1984, 53:116–125.
Golestaneh N, Picaud S, Mirshahi M: The mineralocorticoid receptor in rodent retina: ontogeny and molecular identity. Mol Vis 2002, 8:221–225.
Farman N, Kusch M, Edelman IS: Aldosterone receptor occupancy and sodium transport in the urinary bladder of Bufo marinus. Am J Physiol 1978, 235:C90-C96.
Herkner K, Pollak A, Swoboda W, Holler B: Explanation of the pseudohypoaldosteronism (PHA)-stress syndrome with an artificial aldosterone receptor model. J Steroid Biochem 1984, 20:317–320.
Hatakeyama H, Miyamori I, Fujita T: Vascular aldosterone. Biosynthesis and a link to angiotensin II-induced hypertrophy of vascular smooth muscle cells. J Biol Chem 1994, 269:24316–24320.
Nichols NR, Hall CE, Meyer WJ: Decreased aldosterone receptor affinity in aortic cells from the Fischer 344 rat. J Hypertens 1983, 1:393–397.
Anderson NS III, Fanestil DD: Corticoid receptors in rat brain: evidence for an aldosterone receptor. Endocrinology 1976, 98:676–684.
Moguilewsky M, Raynaud JP: Evidence for a specific mineralocorticoid receptor in rat pituitary and brain. J Steroid Biochem 1980, 12:309–314.
McEwen BS, Lambdin LT, Rainbow TC, De Nicola AF: Aldosterone effects on salt appetite in adrenalectomized rats. Neuroendocrinology 1986, 43:38–43.
Gerlach JL, McEwen BS: Rat brain binds adrenal steroid hormone: radioautography of hippocampus with corticosterone. Science 1972, 175:1133–1136.
De Kloet R, Wallach G, McEwen BS: Differences in corticosterone and dexamethasone binding to rat brain and pituitary. Endocrinology 1975, 96:598–609.
Birmingham MK, Stumpf WE, Sar M: Nuclear localization of aldosterone in rat brain cells assessed by autoradiography. Experientia 1979, 35:1240–1241.
De Nicola AF, Tornello S, Weisenberg L, et al.: Uptake and binding of [3H]aldosterone by the anterior pituitary and brain regions in adrenalectomized rats. Horm Metab Res 1981, 13:103–106.
Schwartz B, Wysocki A: Mineralocorticoid receptors in the rabbit iris-ciliary body. Ophthalmic Res 1997, 29:42–47.
Pratt WB: The role of heat shock proteins in regulating the function, folding, and trafficking of the glucocorticoid receptor. J Biol Chem 1993, 268:21455–21458.
Fanestil DD, Kipnowski J: Molecular action of aldosterone. Klin Wochenschr 1982, 60:1180–1185.
Bhargava A, Fullerton MJ, Myles K, et al.: The serum- and glucocorticoid-induced kinase is a physiological mediator of aldosterone action. Endocrinology 2001, 142:1587–1594.
Granger JP, Kassab S, Novak J, et al.: Role of nitric oxide in modulating renal function and arterial pressure during chronic aldosterone excess. Am J Physiol 1999, 276:R197-R202.
Lombes M, Alfaidy N, Eugene E, et al.: Prerequisite for cardiac aldosterone action. Mineralocorticoid receptor and 11 beta-hydroxysteroid dehydrogenase in the human heart. Circulation 1995, 92:175–182.
Roland BL, Krozowski ZS, Funder JW: Glucocorticoid receptor, mineralocorticoid receptors, 11 beta-hydroxysteroid dehydrogenase-1 and -2 expression in rat brain and kidney: in situ studies. Mol Cell Endocrinol 1995, 111:R1-R7.
Takeda Y, Miyamori I, Inaba S, et al.: Vascular aldosterone in genetically hypertensive rats. Hypertension 1997, 29:45–48.
Christ M, Douwes K, Eisen C, et al.: Rapid effects of aldosterone on sodium transport in vascular smooth muscle cells. Hypertension 1995, 25:117–123.
Alzamora R, Michea L, Marusic ET: Role of 11beta-hydroxysteroid dehydrogenase in nongenomic aldosterone effects in human arteries. Hypertension 2000, 35:1099–1104.
Inglis JA, Halliday JW: Renal damage after subtotal nephrectomy. Pathology 1969, 1:177–183.
Quan ZY, Walser M, Hill GS: Adrenalectomy ameliorates ablative nephropathy in the rat independently of corticosterone maintenance level. Kidney Int 1992, 41:326–333.
Akimov I, Kosithyn NS: Influence of unilateral nephrectomy on aldosterone receptors in the remaining kidney in rats. Izv Akad Nauk Ser Biol 2002, 597–600.
Ullian ME, Gantt BJ, Ford AK, et al.: Potential importance of glomerular citrate synthase activity in remnant nephropathy. Kidney Int 2003, 63:156–164.
Ullian ME, Robinson CJ, Evans CT, et al.: Role of citrate synthase in aldosterone-mediated sodium reabsorption. Hypertension 2000, 35:875–879.
Fitzgibbon WR, Greene EL, Grewal JS, et al.: Resistance to remnant nephropathy in the Wistar-Furth rat. J Am Soc Nephrol 1999, 10:814–821.
Ullian ME, Islam MM, Robinson CJ, et al.: Resistance to mineralocorticoids in Wistar-Furth rats. Am J Physiol 1997, 272:H1454-H1461.
Kenyon CJ, Deconti GA, Cupolo NA, Morris DJ: The role of aldosterone in the development of hypertension in spontaneously hypertensive rats. Endocrinology 1981, 109:1841–1845.
Gavras H, Brunner HR, Laragh JH: Renin and aldosterone and the pathogenesis of hypertensive vascular damage. Prog Cardiovasc Dis 1974, 17:39–49.
Greene EL, Kren S, Hostetter TH: Role of aldosterone in the remnant kidney model in the rat. J Clin Invest 1996, 98:1063–1068.
Rocha R, Chander PN, Khanna K, et al.: Mineralocorticoid blockade reduces vascular injury in stroke-prone hypertensive rats. Hypertension 1998, 31:451–458. Illustrates deleterious role of mineralocorticoids as hormonal mediators of vascular injury and the vascular and end-organ protective effect of spironolactone in an animal model.
Rocha R, Chander PN, Zuckerman A, Stier CT Jr: Role of aldosterone in renal vascular injury in stroke-prone hypertensive rats. Hypertension 1999, 33:232–237. Illustrates deleterious role of aldosterone as a mediator of renal injury despite classical RAAS blockade in an animal model.
Rocha R, Stier CT Jr, Kifor I, et al.: Aldosterone: a mediator of myocardial necrosis and renal arteriopathy. Endocrinology 2000, 141:3871–3878. Offers evidence that aldosterone is a critical mediator of angiotensin II-induced vascular, renal, and cardiac damage in an animal model.
Clore J, Schoolwerth A, Watlington CO: When is cortisol a mineralocorticoid? Kidney Int 1992, 42:1297–1308.
Lifton RP, Dluhy RG, Powers M, et al.: A chimaeric 11 beta-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature 1992, 355:262–265.
Hautanena A, Lankinen L, Kupari M, et al.: Associations between aldosterone synthase gene polymorphism and the adrenocortical function in males. J Intern Med 1998, 244:11–18.
Brand E, Schorr U, Ringel J, et al.: Aldosterone synthase gene (CYP11B2) C-344T polymorphism in Caucasians from the Berlin Salt-Sensitivity Trial (BeSST). J Hypertens 1999, 17:1563–1567.
Patel S, Steeds R, Channer K, Samani NJ: Analysis of promoter region polymorphism in the aldosterone synthase gene (CYP11B2) as a risk factor for myocardial infarction. Am J Hypertens 2000, 13:134–139.
Hengstenberg C, Holmer SR, Mayer B, et al.: Evaluation of the aldosterone synthase (CYP11B2) gene polymorphism in patients with myocardial infarction. Hypertension 2000, 35:704–709.
Hautanen A, Toivanen P, Manttari M, et al.: Joint effects of an aldosterone synthase (CYP11B2) gene polymorphism and classic risk factors on risk of myocardial infarction. Circulation 1999, 100:2213–2218.
Matsubara M, Omori F, Fujita S, et al.: Haplotypes of aldosterone synthase (CYP11B2) gene in the general population of Japan: the Ohasama study. Clin Exp Hypertens 2001, 23:603–610.
Matsubara M, Kikuya M, Ohkubo T, et al.: Aldosterone synthase gene (CYP11B2) C-334T polymorphism, ambulatory blood pressure and nocturnal decline in blood pressure in the general Japanese population: the Ohasama Study. J Hypertens 2001, 19:2179–2184.
Chen A, Zhang W, Tang X, et al.: The relationship of aldosterone synthase gene polymorphism with hypertension and left ventricular hypertrophy. Zhonghua Nei Ke Za Zhi 2002, 41:298–301.
Chen AH, Zhang WX, Li ZL, et al.: Association between aldosterone synthase gene polymorphism and hypertrophic cardiomyopathy. Di Yi Jun Yi Da Xue Xue Bao 2002, 22:704–706.
Lacolley P, Labat C, Pujol A, et al.: Increased carotid wall elastic modulus and fibronectin in aldosterone-salt-treated rats: effects of eplerenone. Circulation 2002, 106:2848–2853.
Neves MF, Virdis A, Schiffrin EL: Resistance artery mechanics and composition in angiotensin II-infused rats: effects of aldosterone antagonism. J Hypertens 2003, 21:189–198.
Ullian ME, Walsh LG, Morinelli TA: Potentiation of angiotensin II action by corticosteroids in vascular tissue. Cardiovasc Res 1996, 32:266–273.
Rocha R, Martin-Berger CL, Yang P, et al.: Selective aldosterone blockade prevents angiotensin II/salt-induced vascular inflammation in the rat heart. Endocrinology 2002, 143:4828–4836.
Suzuki G, Morita H, Mishima T, et al.: Effects of long-term monotherapy with eplerenone, a novel aldosterone blocker, on progression of left ventricular dysfunction and remodeling in dogs with heart failure. Circulation 2002, 106:2967–2972.
Romagni P, Rossi F, Guerrini L, et al.: Aldosterone induces contraction of the resistance arteries in man. Atherosclerosis 2003, 166:345–349.
Pitt B, Zannad F, Remme WJ, et al.: The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999, 341:709–717. First large-scale study in human subjects to prove that aldosterone blockade with spironolactone improves survival and is cardioprotective in patients with CHF.
Pitt B, Remme W, Zannad F, et al.: Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003, 348:1309–1321. First large-scale study in human subjects to prove that eplerenone, the new selective aldosterone receptor antagonist, improves survival and is cardioprotective in patients with CHF.
Ardaillou R: Angiotensin II receptors. J Am Soc Nephrol 1999, 10(11):S30-S39.
Sato A, Suzuki Y, Shibata H, Saruta T: Plasma aldosterone concentrations are not related to the degree of angiotensinconverting enzyme inhibition in essential hypertensive patients. Hypertens Res 2000, 23:25–31. Introduces the concept of aldosterone synthesis escape despite classical RAAS blockade in human subjects by proving that plasma aldosterone concentrations tend to increase with the duration of ACE inhibitor treatment.
Sato A, Hayashi K, Naruse M, Saruta T: Effectiveness of aldosterone blockade in patients with diabetic nephropathy. Hypertension 2003, 41:64–68. First study in the literature to suggest that aldosterone blockade is renoprotective in patients with early diabetic nephropathy who show aldosterone escape during ACE inhibitor treatment and who no longer show maximal antiproteinuric effects of ACE inhibition.
Sato A, Saruta T: Aldosterone escape during angiotensinconverting enzyme inhibitor therapy in essential hypertensive patients with left ventricular hypertrophy. J Int Med Res 2001, 29:13–21. Introduces the concept that aldosterone synthesis escape may reverse the beneficial effects of an ACE inhibitor on left ventricular hypertrophy.
Cicoira M, Zanolla L, Rossi A, et al.: Failure of aldosterone suppression despite angiotensin-converting enzyme (ACE) inhibitor administration in chronic heart failure is associated with ACE DD genotype. J Am Coll Cardiol 2001, 37:1808–1812.
Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N Engl J Med 1987, 316:1429–1435.
Cicoira M, Zanolla L, Franceschini L, et al.: Relation of aldosterone "escape" despite angiotensin-converting enzyme inhibitor administration to impaired exercise capacity in chronic congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 2002, 89:403–407.
Shiigai T, Shichiri M: Late escape from the antiproteinuric effect of ace inhibitors in nondiabetic renal disease. Am J Kidney Dis 2001, 37:477–483.
Li S, Wu P, Zhong S, et al.: Effects of long-term enalapril and losartan therapy of hypertension on cardiovascular aldosterone. Horm Res 2001, 55:293–297.
Xiu JC, Wu P, Xu JP, et al.: Effects of long-term enalapril and losartan therapy of heart failure on cardiovascular aldosterone. J Endocrinol Invest 2002, 25:463–468.
Cohn JN, Tognoni G: A randomized trial of the angiotensinreceptor blocker valsartan in chronic heart failure. N Engl J Med 2001, 345:1667–1675.
Wong M, Staszewsky L, Latini R, et al.: Valsartan benefits left ventricular structure and function in heart failure: Val-HeFT echocardiographic study. J Am Coll Cardiol 2002, 40:970–975.
Agarwal R: Add-on angiotensin receptor blockade with maximized ACE inhibition. Kidney Int 2001, 59:2282–2289.
Zannad F, Alla F, Dousset B, et al.: Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the randomized aldactone evaluation study (RALES). Rales Investigators. Circulation 2000, 102:2700–2706.
Kasama S, Toyama T, Kumakura H, et al.: Spironolactone improves cardiac sympathetic nerve activity and symptoms in patients with congestive heart failure. J Nucl Med 2002, 43:1279–1285.
Cicoira M, Zanolla L, Rossi A, et al.: Long-term, dose-dependent effects of spironolactone on left ventricular function and exercise tolerance in patients with chronic heart failure. J Am Coll Cardiol 2002, 40:304–310.
Tsutamoto T, Wada A, Maeda K, et al.: Effect of spironolactone on plasma brain natriuretic peptide and left ventricular remodeling in patients with congestive heart failure. J Am Coll Cardiol 2001, 37:1228–1233.
Pitt B, Williams G, Remme W, et al.: The EPHESUS trial: eplerenone in patients with heart failure due to systolic dysfunction complicating acute myocardial infarction. Eplerenone Post-AMI Heart Failure Efficacy and Survival Study. Cardiovasc Drugs Ther 2001, 15:79–87.
Spertus JA, Tooley J, Jones P, et al.: Expanding the outcomes in clinical trials of heart failure: the quality of life and economic components of EPHESUS (EPlerenone’s neuro-Hormonal Efficacy and SUrvival Study). Am Heart J 2002, 143:636–642.
Epstein M, Buckalew V, Martinez F, et al.: Antiproteinuric efficacy of eplerenone, enalapril, and eplerenone/enalapril combination in diabetic hypertensives with microalbuminuria [abstract]. Am J Hypertens 2002, 15:24A.
de Gasparo M, Joss U, Ramjoue HP, et al.: Three new epoxy-spirolactone derivatives: characterization in vivo and in vitro. J Pharmacol Exp Ther 1987, 240:650–656.
de Gasparo M, Cumin F, Nussberger J, et al.: Pharmacological investigations of a new renin inhibitor in normal sodiumunrestricted volunteers. Br J Clin Pharmacol 1989, 27:587–596.
Delyani JA: Mineralocorticoid receptor antagonists: the evolution of utility and pharmacology. Kidney Int 2000, 57:1408–1411.
Weinberger MH, Roniker B, Krause SL, Weiss RJ: Eplerenone, a selective aldosterone blocker, in mild-to-moderate hypertension. Am J Hypertens 2002, 15:709–716.
Krum H, Nolly H, Workman D, et al.: Efficacy of eplerenone added to renin-angiotensin blockade in hypertensive patients. Hypertension 2002, 40:117–123. Illustrates the antihypertensive effects of eplerenone and that eplerenone may be useful add-on therapy in hypertensive patients inadequately controlled on an ACE inhibitor or ARB alone.
Krum H, Nolly H, Roniker B, et al.: Coadministration of eplerenone with an angiotensin-converting enzyme inhibitor or an angiotensin II blocker in patients with mild to moderate hypertension (supplement). Eur Heart J 2001, 22:612.
Ibrahim HN, Rosenberg ME, Hostetter TH: Role of the reninangiotensin-aldosterone system in the progression of renal disease: a critical review. Semin Nephrol 1997, 17:431–440.
Remuzzi G, Ruggenenti P, Benigni A: Understanding the nature of renal disease progression. Kidney Int 1997, 51:2–15.
Epstein M: Aldosterone as a mediator of progressive renal dysfunction: evolving perspectives. Intern Med 2001, 40:573–583.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lakkis, J., Lu, W.X. & Weir, M.R. RAAS escape: A real clinical entity that may be important in the progression of cardiovascular and renal disease. Current Science Inc 5, 408–417 (2003). https://doi.org/10.1007/s11906-003-0087-9
Issue Date:
DOI: https://doi.org/10.1007/s11906-003-0087-9