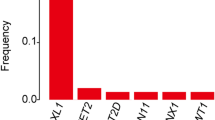
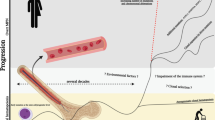
Abstract
Purpose of Review
Although BCR-ABL1-negative myeloproliferative neoplasms (MPN) are chronic, clonal hematopoietic stem cell (HSC) disorders marked by proliferation of one or more myeloid lineages, a substantial proportion of patients transform to acute myeloid leukemia. Leukemic transformation (LT) from a pre-existing MPN carries a dismal prognosis. Here, we review recent genetic, biological, and clinical data regarding LT.
Recent Findings
In the last decade, DNA sequencing has revolutionized our understanding of the genomic landscape of LT. Mutations in TP53, ASXL1, EZH2, IDH1/2, and SRSF2 are significantly associated with increased risk of LT of MPNs. Preclinical modeling of these mutations is underway and has yielded important biological insights, some of which have therapeutic implications.
Summary
Recent progress has led to the identification of recurrent genomic alterations in patients with LT. This has allowed mechanistic and therapeutic insight into the process of LT. In turn, this may lead to more mechanism-based therapeutic strategies that may improve patient outcomes.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Chihara D, Kantarjian HM, Newberry KJ, et al. Survival outcome of patients with acute myeloid leukemia transformed from myeloproliferative neoplasms. Blood. 2016;128(22).
•• Mesa RA, Li CY, Ketterling RP, Schroeder GS, Knudson RA, Tefferi A. Leukemic transformation in myelofibrosis with myeloid metaplasia: a single-institution experience with 91 cases. Blood. 2005;105(3):973–7. This study demonstrates that patients with LT appear to have a poor overall survival regardless of treatment type.
Passamonti F, Rumi E, Arcaini L, Castagnola C, Lunghi M, Bernasconi P, et al. Leukemic transformation of polycythemia vera - a single center study of 23 patients. Cancer. 2005;104(5):1032–6.
Thepot S, Itzykson R, Seegers V, Raffoux E, Quesnel B, Chait Y, et al. Treatment of progression of Philadelphia-negative myeloproliferative neoplasms to myelodysplastic syndrome or acute myeloid leukemia by azacitidine: a report on 54 cases on the behalf of the Groupe Francophone des Myelodysplasies (GFM). Blood. 2010;116(19):3735–42.
Shanavas M, Popat U, Michaelis LC, Fauble V, McLornan D, Klisovic R, et al. Outcomes of allogeneic hematopoietic cell transplantation in patients with myelofibrosis with prior exposure to Janus kinase 1/2 inhibitors. Biol Blood Marrow Transplant. 2016;22(3):432–40.
Mascarenhas J, Navada S, Malone A, Rodriguez A, Najfeld V, Hoffman R. Therapeutic options for patients with myelofibrosis in blast phase. Leuk Res. 2010;34(9):1246–9.
Polliack A, Prokocimer M, Matzner Y. Lymphoblastic leukemic transformation (lymphoblastic crisis) in myelofibrosis and myeloid metaplasia. Am J Hematol. 1980;9(2):211–20.
Cervantes F, Tassies D, Salgado C, Rovira M, Pereira A, Rozman C. Acute transformation in nonleukemic chronic myeloproliferative disorders: actuarial probability and main characteristics in a series of 218 patients. Acta Haematol. 1991;85(3):124–7.
Huang J, Li CY, Mesa RA, Wu W, Hanson CA, Pardanani A, et al. Risk factors for leukemic transformation in patients with primary myelofibrosis. Cancer. 2008;112(12):2726–32.
Bjorkholm M, Derolf AR, Hultcrantz M, et al. Treatment-related risk factors for transformation to acute myeloid leukemia and myelodysplastic syndromes in myeloproliferative neoplasms. J Clin Oncol. 2011;29(17):2410–5.
Finazzi G, Caruso V, Marchioli R, Capnist G, Chisesi T, Finelli C, et al. Acute leukemia in polycythemia vera: an analysis of 1638 patients enrolled in a prospective observational study. Blood. 2005;105(7):2664–70.
Barosi G, Ambrosetti A, Centra A, Falcone A, Finelli C, Foa P, et al. Splenectomy and risk of blast transformation in myelofibrosis with myeloid metaplasia. Italian Cooperative Study Group on Myeloid with Myeloid Metaplasia. Blood. 1998;91(10):3630–6.
Barosi G, Ambrosetti A, Buratti A, Finelli C, Liberato NL, Quaglini S, et al. Splenectomy for patients with myelofibrosis with myeloid metaplasia: pretreatment variables and outcome prediction. Leukemia. 1993;7(2):200–6.
Lafaye F, Rain JD, Clot P, Najean Y. Risks and benefits of splenectomy in myelofibrosis: an analysis of 39 cases. Nouv Rev Fr Hematol. 1994;36(5):359–62.
Tam CS, Nussenzveig RM, Popat U, Bueso-Ramos CE, Thomas DA, Cortes JA, et al. The natural history and treatment outcome of blast phase BCR-ABL-myeloproliferative neoplasms. Blood. 2008;112(5):1628–37.
Tefferi A, Mesa RA, Nagorney DM, Schroeder G, Silverstein MN. Splenectomy in myelofibrosis with myeloid metaplasia: a single-institution experience with 223 patients. Blood. 2000;95(7):2226–33.
Tam CS, Kantarjian H, Cortes J, Lynn A, Pierce S, Zhou L, et al. Dynamic model for predicting death within 12 months in patients with primary or post-polycythemia vera/essential thrombocythemia myelofibrosis. J Clin Oncol. 2009;27(33):5587–93.
Fruchtman SM, Mack K, Kaplan ME, Peterson P, Berk PD, Wasserman LR. From efficacy to safety: a Polycythemia Vera Study group report on hydroxyurea in patients with polycythemia vera. Semin Hematol. 1997;34(1):17–23.
Barbui T. The leukemia controversy in myeloproliferative disorders: is it a natural progression of disease, a secondary sequela of therapy, or a combination of both? Semin Hematol. 2004;41(2 Suppl 3):15–7.
Barbui T, Carobbio A, Finazzi G, Vannucchi AM, Barosi G, Antonioli E, et al. Inflammation and thrombosis in essential thrombocythemia and polycythemia vera: different role of C-reactive protein and pentraxin 3. Haematologica. 2011;96(2):315–8.
Tefferi A, Guglielmelli P, Larson DR, Finke C, Wassie EA, Pieri L, et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood. 2014;124(16):2507–13.
Kent DG, Li J, Tanna H, et al. Self-renewal of single mouse hematopoietic stem cells is reduced by JAK2V617F without compromising progenitor cell expansion. Plos Biology. 2013;11(6).
Li J, Spensberger D, Ahn JS, Anand S, Beer PA, Ghevaert C, et al. JAK2 V617F impairs hematopoietic stem cell function in a conditional knock-in mouse model of JAK2 V617F-positive essential thrombocythemia. Blood. 2010;116(9):1528–38.
Mullally A, Lane SW, Ball B, Megerdichian C, Okabe R, al-Shahrour F, et al. Physiological Jak2V617F expression causes a lethal myeloproliferative neoplasm with differential effects on hematopoietic stem and progenitor cells. Cancer Cell. 2010;17(6):584–96.
Ishii T, Zhao Y, Sozer S, Shi J, Zhang W, Hoffman R, et al. Behavior of CD34+ cells isolated from patients with polycythemia vera in NOD/SCID mice. Exp Hematol. 2007;35(11):1633–40.
James C, Mazurier F, Dupont S, Chaligne R, Lamrissi-Garcia I, Tulliez M, et al. The hematopoietic stem cell compartment of JAK2V617F-positive myeloproliferative disorders is a reflection of disease heterogeneity. Blood. 2008;112(6):2429–38.
•• Vannucchi AM, Lasho TL, Guglielmelli P, et al. Mutations and prognosis in primary myelofibrosis. Leukemia. 2013;27(9):1861–9. This study describes the specific mutations associated with the highest risk of LT.
Lundberg P, Karow A, Nienhold R, Looser R, Hao-Shen H, Nissen I, et al. Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood. 2014;123(14):2220–8.
Wang L, Swierczek SI, Drummond J, Hickman K, Kim SJ, Walker K, et al. Whole-exome sequencing of polycythemia vera revealed novel driver genes and somatic mutation shared by T cells and granulocytes. Leukemia. 2014;28(4):935–8.
Delic S, Rose D, Kern W, Nadarajah N, Haferlach C, Haferlach T, et al. Application of an NGS-based 28-gene panel in myeloproliferative neoplasms reveals distinct mutation patterns in essential thrombocythaemia, primary myelofibrosis and polycythaemia vera. Br J Haematol. 2016;175(3):419–26.
Lasho TL, Jimma T, Finke CM, Patnaik M, Hanson CA, Ketterling RP, et al. SRSF2 mutations in primary myelofibrosis: significant clustering with IDH mutations and independent association with inferior overall and leukemia-free survival. Blood. 2012;120(20):4168–71.
Courtier F, Carbuccia N, Garnier S, Guille A, Adélaïde J, Cervera N, et al. Genomic analysis of myeloproliferative neoplasms in chronic and acute phases. Haematologica. 2017;102(1):e11–4.
Li B, Gale RP, Xu Z, Qin T, Song Z, Zhang P, et al. Non-driver mutations in myeloproliferative neoplasm-associated myelofibrosis. J Hematol Oncol. 2017;10(1):99.
•• Rampal R, Ahn J, Abdel-Wahab O, et al. Genomic and functional analysis of leukemic transformation of myeloproliferative neoplasms. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(50):E5401-E5410. This was the description of a JAK2V617F driven murine leukemia model. The study also contains sequencing information from paired MPN and LT samples.
Harutyunyan A, Klampfl T, Cazzola M, Kralovics R. p53 lesions in leukemic transformation. N Engl J Med. 2011;364(5):488–90.
Zoi K, Cross NC. Genomics of myeloproliferative neoplasms. J Clin Oncol. 2017;35(9):947–54.
Sabapathy K, Lane DP. Therapeutic targeting of p53: all mutants are equal, but some mutants are more equal than others. Nat Rev Clin Oncol. 2018;15(1):13–30.
Abdel-Wahab O, Manshouri T, Patel J, Harris K, Yao J, Hedvat C, et al. Genetic analysis of transforming events that convert chronic myeloproliferative neoplasms to leukemias. Cancer Res. 2010;70(2):447–52.
Kim E, Ilagan JO, Liang Y, Daubner GM, Lee SCW, Ramakrishnan A, et al. SRSF2 mutations contribute to myelodysplasia by mutant-specific effects on exon recognition. Cancer Cell. 2015;27(5):617–30.
Ortmann CA, Kent DG, Nangalia J, Silber Y, Wedge DC, Grinfeld J, et al. Effect of mutation order on myeloproliferative neoplasms. N Engl J Med. 2015;372(7):601–12.
Chen E, Schneider RK, Breyfogle LJ, Rosen EA, Poveromo L, Elf S, et al. Distinct effects of concomitant Jak2V617F expression and Tet2 loss in mice promote disease progression in myeloproliferative neoplasms. Blood. 2015;125(2):327–35.
Kameda T, Shide K, Yamaji T, Kamiunten A, Sekine M, Taniguchi Y, et al. Loss of TET2 has dual roles in murine myeloproliferative neoplasms: disease sustainer and disease accelerator. Blood. 2015;125(2):304–15.
Tefferi A, Jimma T, Sulai NH, Lasho TL, Finke CM, Knudson RA, et al. IDH mutations in primary myelofibrosis predict leukemic transformation and shortened survival: clinical evidence for leukemogenic collaboration with JAK2V617F. Leukemia. 2012;26(3):475–80.
Pardanani A, Lasho TL, Finke CM, Mai M, McClure RF, Tefferi A. IDH1 and IDH2 mutation analysis in chronic- and blast-phase myeloproliferative neoplasms. Leukemia. 2010;24(6):1146–51.
Sasaki M, Knobbe CB, Munger JC, et al. IDH1(R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature. 2012;488(7413):656−+.
Chen C, Liu Y, Lu C, Cross JR, Morris JP, Shroff AS, et al. Cancer-associated IDH2 mutants drive an acute myeloid leukemia that is susceptible to Brd4 inhibition. Genes Dev. 2013;27(18):1974–85.
Chaturvedi A, Cruz MMA, Jyotsana N, et al. Mutant IDH1 promotes leukemogenesis in vivo and can be specifically targeted in human AML. Blood. 2013;122(16):2877–87.
McKenney AS, Allison N, Somasundara AVH, et al. JAK2/IDH-mutant-driven myeloproliferative neoplasm is sensitive to combined targeted inhibition. J Clin Investig. 2018;128(2):789–804.
Lasho TL, Mudireddy M, Finke CM, Hanson CA, Ketterling RP, Szuber N, et al. Targeted next-generation sequencing in blast phase myeloproliferative neoplasms. Blood Adv. 2018;2(4):370–80.
Abdel-Wahab O, Gao J, Adli M, Dey A, Trimarchi T, Chung YR, et al. Deletion of Asxl1 results in myelodysplasia and severe developmental defects in vivo. J Exp Med. 2013;210(12):2641–59.
Wang J, Li Z, He Y, Pan F, Chen S, Rhodes S, et al. Loss of Asxl1 leads to myelodysplastic syndrome-like disease in mice. Blood. 2014;123(4):541–53.
Abdel-Wahab O, Adli M, LaFave LM, et al. ASXL1 mutations promote myeloid transformation through loss of PRC2-mediated gene repression. Cancer Cell. 2012;22(2):180–93.
Yang Y, Akada H, Nath D, Hutchison RE, Mohi G. Loss of Ezh2 cooperates with Jak2V617F in the development of myelofibrosis in a mouse model of myeloproliferative neoplasm. Blood. 2016;127(26):3410–23.
Shimizu T, Kubovcakova L, Nienhold R, Zmajkovic J, Meyer SC, Hao-Shen H, et al. Loss of Ezh2 synergizes with JAK2-V617F in initiating myeloproliferative neoplasms and promoting myelofibrosis. J Exp Med. 2016;213(8):1479–96.
Zhang SJ, Rampal R, Manshouri T, Patel J, Mensah N, Kayserian A, et al. Genetic analysis of patients with leukemic transformation of myeloproliferative neoplasms shows recurrent SRSF2 mutations that are associated with adverse outcome. Blood. 2012;119(19):4480–5.
Granfeldt Ostgard LS, Medeiros BC, Sengelov H, et al. Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: a National Population-Based Cohort Study. J Clin Oncol. 2015;33(31):3641–9.
•• Kennedy JA, Atenafu EG, Messner HA, et al. Treatment outcomes following leukemic transformation in Philadelphia-negative myeloproliferative neoplasms. Blood. 2013;121(14):2725–33. This study demonstrates that patients with LT treated with induction chemotherapy may derive benefit if they receive allogeneic stem cell transplant. However, patients who achieve a remission and don't receive transplant may not derive benefit from induction chemotherapy.
Cherington C, Slack JL, Leis J, Adams RH, Reeder CB, Mikhael JR, et al. Allogeneic stem cell transplantation for myeloproliferative neoplasm in blast phase. Leuk Res. 2012;36(9):1147–51.
Eghtedar A, Verstovsek S, Estrov Z, Burger J, Cortes J, Bivins C, et al. Phase 2 study of the JAK kinase inhibitor ruxolitinib in patients with refractory leukemias, including postmyeloproliferative neoplasm acute myeloid leukemia. Blood. 2012;119(20):4614–8.
Mwirigi A, Galli S, Keohane C, Raj K, Radia DH, Harrison CN, et al. Combination therapy with ruxolitinib plus 5-azacytidine or continuous infusion of low dose cytarabine is feasible in patients with blast-phase myeloproliferative neoplasms. Br J Haematol. 2014;167(5):714–6.
Devillier R, Raffoux E, Rey J, Lengline E, Ronchetti AM, Sebert M, et al. Combination therapy with ruxolitinib plus intensive treatment strategy is feasible in patients with blast-phase myeloproliferative neoplasms. Br J Haematol. 2016;172(4):628–30.
Rampal Rea. Safety and efficacy of combined ruxolitinib and decitabine in patients with blast-phase MPN and post-MPN AML: results of a phase I study (myeloproliferative disorders research consortium 109 trial). American Society of Hematology annual meeting abstract 2016.
al. Be. Phase I/II study of ruxolitinib (RUX) with decitabine (DAC) in patients with post-myeloproliferative neoplasm acute myeloid leukemia (post-MPN AML): phase I results. American Society of Hematology annual meeting. 2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Bing Li declares no conflict of interest.
John O. Mascarenhas reports grants from Incyte, Novartis, Janssen, Roche, CTI Biopharma, Pharmaessentia, Celgen, Merck, and Promedior.
Raajit K. Rampal reports personal fees from Incyte, Celgene, Jazz, Apexx, and Agios and grants from Constellation and Stemline.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Myeloproliferative Neoplasms
Rights and permissions
About this article
Cite this article
Li, B., Mascarenhas, J.O. & Rampal, R.K. Leukemic Transformation of Myeloproliferative Neoplasms: Therapeutic and Genomic Considerations. Curr Hematol Malig Rep 13, 588–595 (2018). https://doi.org/10.1007/s11899-018-0491-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-018-0491-5