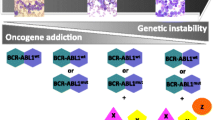
Abstract
The introduction of imatinib represented a breakthrough in the treatment of chronic myelogenous leukemia (CML). However, about 20% of patients treated in early chronic-phase CML are off therapy after 6 years because of resistance or intolerance, and most patients taking imatinib remain BCR-ABL-positive at the molecular level, indicating primary refractoriness of a leukemic subpopulation. Patients with advanced disease often do not respond, or they eventually relapse. Resistance frequently is associated with mutations in the kinase domain of BCR-ABL. Other mechanisms leading to reactivation of BCR-ABL or preventing sufficient BCR-ABL inhibition also exist. Resistance of patients with continued BCR-ABL inhibition despite leukemic progression indicates clonal evolution triggered by BCR-ABL-independent mechanisms. Current efforts to optimize BCR-ABL-targeted treatment focus on the difficulty in reaching CML stem cells. Success will most likely depend on integration of combined treatment algorithms—whether they be a combination of molecules interfering with signaling pathways or additional immune-based treatment adjuncts.
Similar content being viewed by others
References and Recommended Reading
Wong S, Witte ON: The BCR-ABL story: bench to beside and back. Annu Rev Immunol 2004, 22:247–306.
Deininger M, Buchdunger E, Druker BJ: The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood 2005, 105:2640–2653.
Druker BJ, Guilhot F, O’Brien SG, et al.: Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med 2006, 355:2408–2417.
Hochhaus A, Druker B, Sawyers C, et al.: Favorable long-term follow-up results over 6 years for response, survival, and safety with imatinib mesylate therapy in chronic-phase chronic myeloid leukemia after failure of interferon-alpha treatment. Blood 2008, 111:1039–1043.
Cortes J, O’Brien S, Kantarjian H: Discontinuation of imatinib therapy after achieving a molecular response. Blood 2004, 104:2204–2205.
Hughes TP, Kaeda J, Branford S, et al.: Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med 2003, 349:1423–1432.
Baccarani M, Saglio G, Goldman J, et al.: Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood 2006, 108:1809–1820.
Branford S, Seymour JF, Grigg A, et al.: BCR-ABL messenger RNA levels continue to decline in patients with chronic phase chronic myeloid leukemia treated with imatinib for more than 5 years and approximately half of all first-line treated patients have stable undetectable BCR-ABL using strict sensitivity criteria. Clin Cancer Res 2007, 13:7080–7085.
Hughes T, Deininger M, Hochhaus A, et al.: Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood 2006, 108:28–37.
Talpaz M, Silver RT, Druker BJ, et al.: Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: results of a phase 2 study. Blood 2002, 99:1928–1937.
Sawyers CL, Hochhaus A, Feldman E, et al.: Imatinib induces hematologic and cytogenetic responses in patients with chronic myelogenous leukemia in myeloid blast crisis: results of a phase II study. Blood 2002, 99:3530–3539.
White D, Saunders V, Grigg A, et al.: Measurement of in vivo BCR-ABL kinase inhibition to monitor imatinib-induced target blockade and predict response in chronic myeloid leukemia. J Clin Oncol 2007, 25:4445–4451.
Hochhaus A, Kreil S, Corbin AS, et al.: Molecular and chromosomal mechanisms of resistance to imatinib (STI571) therapy. Leukemia 2002, 16:2190–2196.
Gorre ME, Mohammed M, Ellwood K, et al.: Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science 2001, 293:876–880.
Schindler T, Bornmann W, Pellicena P, et al.: Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science 2000, 289:1938–1942.
von Bubnoff N, Schneller F, Peschel C, Duyster J: BCR-ABL gene mutations in relation to clinical resistance of Philadelphia-chromosome-positive leukaemia to STI571: a prospective study. Lancet 2002, 359:487–491.
Branford S, Rudzki Z, Walsh S, et al.: High frequency of point mutations clustered within the adenosine triphosphate-binding region of BCR/ABL in patients with chronic myeloid leukemia or Ph-positive acute lymphoblastic leukemia who develop imatinib (STI571) resistance. Blood 2002, 99:3472–3475.
Branford S, Rudzki Z, Walsh S, et al.: The detection of BCR-ABL mutations in patients with CML treated with imatinib is virtually always accompanied by clinical resistance, and mutations in the ATP phosphate-binding loop (P-loop) are associated with a poor prognosis. Blood 2003, 102:276–283.
Hochhaus A, Kreil S, Corbin A, et al.: Roots of clinical resistance to STI-571 cancer therapy. Science 2001, 293:2163.
Shah NP, Nicoll JM, Nagar B, et al.: Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell 2002, 2:117–125.
Apperley JF: Part I: mechanisms of resistance to imatinib in chronic myeloid leukaemia. Lancet Oncol 2007, 8:1018–1029.
O’Hare T, Eide CA, Deininger MW: Bcr-Abl kinase domain mutations, drug resistance, and the road to a cure for chronic myeloid leukemia. Blood 2007, 110:2242–2249.
Jabbour E, Kantarjian H, Jones D, et al.: Frequency and clinical significance of BCR-ABL mutations in patients with chronic myeloid leukemia treated with imatinib mesylate. Leukemia 2006, 20:1767–1773.
Soverini S, Colarossi S, Gnani A, et al.: Contribution of ABL kinase domain mutations to imatinib resistance in different subsets of Philadelphia-positive patients: by the GIMEMA Working Party on Chronic Myeloid Leukemia. Clin Cancer Res 2006, 12:7374–7379.
Barnes DJ, Palaiologou D, Panousopoulou E, et al.: Bcr-Abl expression levels determine the rate of development of resistance to imatinib mesylate in chronic myeloid leukemia. Cancer Res 2005, 65:8912–8919.
Koptyra M, Falinski R, Nowicki MO, et al.: BCR/ABL kinase induces self-mutagenesis via reactive oxygen species to encode imatinib resistance. Blood 2006, 108:319–327.
Weisberg E, Manley PW, Breitenstein W, et al.: Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell 2005, 7:129–141. [Published erratum appears in Cancer Cell 2005, 7:399.]
Shah NP, Tran C, Lee FY, et al.: Overriding imatinib resistance with a novel ABL kinase inhibitor. Science 2004, 305:399–401.
Weisberg E, Manley PW, Cowan-Jacob SW, et al.: Second generation inhibitors of BCR-ABL for the treatment of imatinib-resistant chronic myeloid leukaemia. Nat Rev Cancer 2007, 7:345–356.
Corbin AS, La Rosée P, Stoffregen EP, et al.: Several Bcr-Abl kinase domain mutants associated with imatinib mesylate resistance remain sensitive to imatinib. Blood 2003, 101:4611–4614.
Griswold IJ, MacPartlin M, Bumm T, et al.: Kinase domain mutants of Bcr-Abl exhibit altered transformation potency, kinase activity, and substrate utilization, irrespective of sensitivity to imatinib. Mol Cell Biol 2006, 26:6082–6093.
Azam M, Latek RR, Daley GQ: Mechanisms of autoinhibition and STI-571/imatinib resistance revealed by mutagenesis of BCR-ABL. Cell 2003, 112:831–843.
von Bubnoff N, Barwisch S, Speicher MR, et al.: A cell-based screening strategy that predicts mutations in oncogenic tyrosine kinases: implications for clinical resistance in targeted cancer treatment. Cell Cycle 2005, 4:400–406.
Bradeen HA, Eide CA, O’Hare T, et al.: Comparison of imatinib mesylate, dasatinib (BMS-354825), and nilotinib (AMN107) in an N-ethyl-N-nitrosourea (ENU)-based mutagenesis screen: high efficacy of drug combinations. Blood 2006, 108:2332–2338.
Roche-Lestienne C, Soenen-Cornu V, Grardel-Duflos N, et al.: Several types of mutations of the Abl gene can be found in chronic myeloid leukemia patients resistant to STI571, and they can pre-exist to the onset of treatment. Blood 2002, 100:1014–1018.
Ernst T, Erben P, Müller MC, et al.: Dynamics of BCR-ABL mutated clones prior to hematologic or cytogenetic resistance to imatinib. Haematologica 2008, 93:186–192.
Branford S, Rudzki Z, Parkinson I, et al.: Real-time quantitative PCR analysis can be used as a primary screen to identify patients with CML treated with imatinib who have BCR-ABL kinase domain mutations. Blood 2004, 104:2926–2932.
Sherbenou DW, Wong MJ, Humayun A, et al.: Mutations of the BCR-ABL-kinase domain occur in a minority of patients with stable complete cytogenetic response to imatinib. Leukemia 2007, 21:489–493.
Khorashad JS, Anand M, Marin D, et al.: The presence of a BCR-ABL mutant allele in CML does not always explain clinical resistance to imatinib. Leukemia 2006, 20:658–663.
Kantarjian H, Talpaz M, O’Brien S, et al.: High-dose imatinib mesylate therapy in newly diagnosed Philadelphia chromosome-positive chronic phase chronic myeloid leukemia. Blood 2004, 103:2873–2878.
Holtz MS, Slovak ML, Zhang F, et al.: Imatinib mesylate (STI571) inhibits growth of primitive malignant progenitors in chronic myelogenous leukemia through reversal of abnormally increased proliferation. Blood 2002, 99:3792–3800.
Wu EQ, Feng W, Johnson S, et al.: Medical costs associated with imatinib (IM) non-compliance in chronic myeloid leukemia (CML) patients (pts) [abstract 17514]. Presented at the 2007 ASCO Annual Meeting. Chicago: May 30–June 3, 2007.
Mahon FX, Deininger MW, Schultheis B, et al.: Selection and characterization of BCR-ABL positive cell lines with differential sensitivity to the tyrosine kinase inhibitor STI571: diverse mechanisms of resistance. Blood 2000, 96:1070–1079.
Brendel C, Scharenberg C, Dohse M, et al.: Imatinib mesylate and nilotinib (AMN107) exhibit high-affinity interaction with ABCG2 on primitive hematopoietic stem cells. Leukemia 2007, 21:1267–1275.
Thomas J, Wang L, Clark RE, Primohamed M: Active transport of imatinib into and out of cells: implications for drug resistance. Blood 2004, 104:3739–3745.
White DL, Saunders VA, Dang P, et al.: OCT-1-mediated influx is a key determinant of the intracellular uptake of imatinib but not nilotinib (AMN107): reduced OCT-1 activity is the cause of low in vitro sensitivity to imatinib. Blood 2006, 108:697–704.
Jiang X, Zhao Y, Smith C, et al.: Chronic myeloid leukemia stem cells possess multiple unique features of resistance to BCR-ABL targeted therapies. Leukemia 2007, 21:926–935.
Picard S, Titier K, Etienne G, et al.: Trough imatinib plasma levels are associated with both cytogenetic and molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood 2007, 109:3496–3499.
O’Dwyer ME, Mauro MJ, Kurilik G, et al.: The impact of clonal evolution on response to imatinib mesylate (STI571) in accelerated phase CML. Blood 2002, 100:1628–1633.
Wendel HG, de Stanchina E, Cepero E, et al.: Loss of p53 impedes the antileukemic response to BCR-ABL inhibition. Proc Natl Acad Sci U S A 2006, 103:7444–7449.
Wang Y, Cai D, Brendel C, et al.: Adaptive secretion of granulocyte-macrophage colony-stimulating factor (GM-CSF) mediates imatinib and nilotinib resistance in BCR/ABL+ progenitors via JAK-2/STAT-5 pathway activation. Blood 2007, 109:2147–2155.
Chu S, Holtz M, Gupta M, Bhatia R: BCR/ABL kinase inhibition by imatinib mesylate enhances MAP kinase activity in chronic myelogenous leukemia CD34+ cells. Blood 2004, 103:3167–3174.
Burchert A, Wang Y, Cai D, et al.: Compensatory PI3-kinase/Akt/mTor activation regulates imatinib resistance development. Leukemia 2005, 19:1774–1782.
Donato NJ, Wu JY, Stapley J, et al.: BCR-ABL independence and LYN kinase overexpression in chronic myelogenous leukemia cells selected for resistance to STI571. Blood 2003, 101:690–698.
Chu S, Xu H, Shah NP, et al.: Detection of BCR-ABL kinase mutations in CD34+ cells from chronic myelogenous leukemia patients in complete cytogenetic remission on imatinib mesylate treatment. Blood 2005, 105:2093–2098.
Graham SM, Jorgensen HG, Allan E, et al.: Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood 2002, 99:319–325.
Rousselot P, Huguet F, Rea D, et al.: Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood 2007, 109:58–60.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rosée, P.L., Hochhaus, A. Resistance to imatinib in chronic myelogenous leukemia: Mechanisms and clinical implications. Curr Hematol Malig Rep 3, 72–79 (2008). https://doi.org/10.1007/s11899-008-0012-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-008-0012-z