Abstract
Purpose of Review
The loss of contractile function after heart injury remains one of the major healthcare issues of our time. One strategy to deal with this problem would be to increase the number of cardiomyocytes to enhance cardiac function. In the last couple of years, reactivation of cardiomyocyte proliferation has repeatedly demonstrated to aid in functional recovery after cardiac injury.
Recent Findings
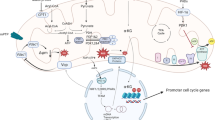
The Tgf-β superfamily plays key roles during development of the heart and populating the embryonic heart with cardiomyocytes. In this review, we discuss the role of Tgf-β signaling in regulating cardiomyocyte proliferation during development and in the setting of cardiac regeneration.
Summary
Although various pathways to induce cardiomyocyte proliferation have been established, the extent to which cardiomyocyte proliferation requires or involves activation of the Tgf-β superfamily is not entirely clear. More research is needed to better understand cross-talk between pathways that regulate cardiomyocyte proliferation.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Benjamin EJ, Muntner P, Bittencourt MS. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–e528.
Konkel L. Assessing a medley of metals: combined exposures and incident coronary heart disease. Environ Health Perspect. 2018;126(3):034002.
Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–44.
Schaufelberger M, Swedberg K, Köster M, Rosén M, Rosengren A. Decreasing one-year mortality and hospitalization rates for heart failure in Sweden: data from the Swedish hospital discharge registry 1988 to 2000. Eur Heart J. 2004;25(4):300–7.
Van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin S-CJ, et al. C-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509(7500):337–41.
Neidig LE, Weinberger F, Palpant NJ, Mignone J, Martinson AM, Sorensen DW, et al. Evidence for minimal cardiogenic potential of stem cell antigen 1–positive cells in the adult mouse heart. Circulation. 2018;138(25):2960–2.
Weinberger F, Eschenhagen T. Heart Regeneration: From Mouse to Human. Current Opinion in Physiology. 2019.
Kretzschmar K, Post Y, Bannier-Hélaouët M, Mattiotti A, Drost J, Basak O, et al. Profiling proliferative cells and their progeny in damaged murine hearts. Proc Natl Acad Sci. 2018;115(52):E12245–E54.
Uygur A, Lee RT. Mechanisms of cardiac regeneration. Dev Cell. 2016;36(4):362–74.
Foglia MJ, Poss KD. Building and re-building the heart by cardiomyocyte proliferation. Development. 2016;143(5):729–40.
Liao S, Dong W, Lv L, Guo H, Yang J, Zhao H, et al. Heart regeneration in adult Xenopus tropicalis after apical resection. Cell Biosci. 2017;7(1):70.
Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331(6020):1078–80.
Mollova M, Bersell K, Walsh S, Savla J, Das LT, Park S-Y, et al. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc Natl Acad Sci. 2013;110(4):1446–51.
Bersell K, Arab S, Haring B, Kühn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138(2):257–70.
von Gise A, Lin Z, Schlegelmilch K, Honor LB, Pan GM, Buck JN, et al. YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proc Natl Acad Sci. 2012;109(7):2394–9.
Xin M, Kim Y, Sutherland LB, Qi X, McAnally J, Schwartz RJ, et al. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci Signal. 2011;4(196):ra70.
Mohamed TM, Ang Y-S, Radzinsky E, Zhou P, Huang Y, Elfenbein A, et al. Regulation of cell cycle to stimulate adult cardiomyocyte proliferation and cardiac regeneration. Cell. 2018;173(1):104–16 e12.
Todorovic V, Jurukovski V, Chen Y, Fontana L, Dabovic B, Rifkin D. Latent TGF-β binding proteins. Int J Biochem Cell Biol. 2005;37(1):38–41.
Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, et al. Latent TGF-β structure and activation. Nature. 2011;474(7351):343–9.
Zhang YE, Newfeld SJ. Meeting report–TGF-β superfamily: signaling in development and disease. The Company of Biologists Ltd; 2013.
Moustakas A, Heldin C-H. The regulation of TGFβ signal transduction. Development. 2009;136(22):3699–714.
Zhang YE. Non-Smad signaling pathways of the TGF-β family. Cold Spring Harb Perspect Biol. 2017;9(2):a022129.
Uribe V, Ramadass R, Dogra D, Rasouli SJ, Gunawan F, Nakajima H, et al. In vivo analysis of cardiomyocyte proliferation during trabeculation. Development. 2018;145(14):dev164194.
Wu C-C, Kruse F, Vasudevarao MD, Junker JP, Zebrowski DC, Fischer K, et al. Spatially resolved genome-wide transcriptional profiling identifies BMP signaling as essential regulator of zebrafish cardiomyocyte regeneration. Dev Cell. 2016;36(1):36–49.
Prados B, Gómez-Apiñániz P, Papoutsi T, Luxán G, Zaffran S, Pérez-Pomares JM, et al. Myocardial Bmp2 gain causes ectopic EMT and promotes cardiomyocyte proliferation and immaturity. Cell Death Dis. 2018;9(3):1–15.
Ebelt H, Hillebrand I, Arlt S, Zhang Y, Kostin S, Neuhaus H, et al. Treatment with bone morphogenetic protein 2 limits infarct size after myocardial infarction in mice. Shock. 2013;39(4):353–60.
Chakraborty S, Sengupta A, Yutzey KE. Tbx20 promotes cardiomyocyte proliferation and persistence of fetal characteristics in adult mouse hearts. J Mol Cell Cardiol. 2013;62:203–13.
Chen H, Shi S, Acosta L, Li W, Lu J, Bao S, et al. BMP10 is essential for maintaining cardiac growth during murine cardiogenesis. Development. 2004;131(9):2219–31.
Heldin C-H, Moustakas A. Signaling receptors for TGF-β family members. Cold Spring Harb Perspect Biol. 2016;8(8):a022053.
Ten Dijke P, Goumans MJ, Itoh F, Itoh S. Regulation of cell proliferation by Smad proteins. J Cell Physiol. 2002;191(1):1–16.
Kennedy BA, Deatherage DE, Gu F, Tang B, Chan MW, Nephew KP, et al. ChIP-seq defined genome-wide map of TGFβ/SMAD4 targets: implications with clinical outcome of ovarian cancer. PLoS One. 2011;6(7):e22606.
Timberlake AT, Choi J, Zaidi S, Lu Q, Nelson-Williams C, Brooks ED, et al. Two locus inheritance of non-syndromic midline craniosynostosis via rare SMAD6 and common BMP2 alleles. Elife. 2016;5:e20125.
Engel FB, Hsieh PC, Lee RT, Keating MT. FGF1/p38 MAP kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proc Natl Acad Sci. 2006;103(42):15546–51.
Maillet M, Purcell NH, Sargent MA, York AJ, Bueno OF, Molkentin JD. DUSP6 (MKP3) null mice show enhanced ERK1/2 phosphorylation at baseline and increased myocyte proliferation in the heart affecting disease susceptibility. J Biol Chem. 2008;283(45):31246–55.
Khalil N. TGF-β: from latent to active. Microbes Infect. 1999;1(15):1255–63.
Bettinger DA, Yager DR, Diegelmann RF, Cohen IK. The effect of TGF-beta on keloid fibroblast proliferation and collagen synthesis. Plast Reconstr Surg. 1996;98(5):827–33.
Saltis J, Agrotis A, Bobik A. TGF-beta 1 potentiates growth factor-stimulated proliferation of vascular smooth muscle cells in genetic hypertension. Am J Phys Cell Phys. 1992;263(2):C420–C8.
Huang SS, Huang JS. TGF-β control of cell proliferation. J Cell Biochem. 2005;96(3):447–62.
Takehara K, LeRoy EC, Grotendorst GR. TGF-β inhibition of endothelial cell proliferation: alteration of EGF binding and EGF-induced growth-regulatory (competence) gene expression. Cell. 1987;49(3):415–22.
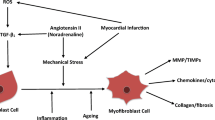
Bujak M, Frangogiannis NG. The role of TGF-β signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74(2):184–95.
Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, et al. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature. 1992;359(6397):693–9.
Proetzel G, Pawlowski SA, Wiles MV, Yin M, Boivin GP, Howles PN, et al. Transforming growth factor–β3 is required for secondary palate fusion. Nat Genet. 1995;11(4):409–14.
Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, et al. Abnormal lung development and cleft palate in mice lacking TGF–β3 indicates defects of epithelial–mesenchymal interaction. Nat Genet. 1995;11(4):415–21.
Stanford L, Ormsby I, Gittenberger-de Groot A, Sariola H, Friedman R. TGFb2 knockout mice have multiple developmental defects that are non-overlapping with other TGFb phenotypes. Development. 1997;124:2569–670.
McKoy G, Bicknell KA, Patel K, Brooks G. Developmental expression of myostatin in cardiomyocytes and its effect on foetal and neonatal rat cardiomyocyte proliferation. Cardiovasc Res. 2007;74(2):304–12.
Cohn RD, Liang H-Y, Shetty R, Abraham T, Wagner KR. Myostatin does not regulate cardiac hypertrophy or fibrosis. Neuromuscul Disord. 2007;17(4):290–6.
Heineke J, Auger-Messier M, Xu J, Sargent M, York A, Welle S, et al. Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure. Circulation. 2010;121(3):419–25.
Sridurongrit S, Larsson J, Schwartz R, Ruiz-Lozano P, Kaartinen V. Signaling via the Tgf-β type I receptor Alk5 in heart development. Dev Biol. 2008;322(1):208–18.
Inman GJ, Nicolás FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, et al. SB-431542 is a potent and specific inhibitor of transforming growth factor-β superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62(1):65–74.
Chablais F, Jaźwińska A. The regenerative capacity of the zebrafish heart is dependent on TGFβ signaling. Development. 2012;139(11):1921–30.
Pfefferli C, Jaźwińska A. The careg element reveals a common regulation of regeneration in the zebrafish myocardium and fin. Nat Commun. 2017;8(1):1–16.
Snider P, Standley KN, Wang J, Azhar M, Doetschman T, Conway SJ. Origin of cardiac fibroblasts and the role of periostin. Circ Res. 2009;105(10):934–47.
Kühn B, Del Monte F, Hajjar RJ, Chang Y-S, Lebeche D, Arab S, et al. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007;13(8):962–9.
Lorts A, Schwanekamp JA, Elrod JW, Sargent MA, Molkentin JD. Genetic manipulation of periostin expression in the heart does not affect myocyte content, cell cycle activity, or cardiac repair. Circ Res. 2009;104(1):e1–7.
López-Novoa JM, Bernabeu C. The physiological role of endoglin in the cardiovascular system. Am J Phys Heart Circ Phys. 2010;299(4):H959–H74.
Arthur HM, Ure J, Smith AJ, Renforth G, Wilson DI, Torsney E, et al. Endoglin, an ancillary TGFβ receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev Biol. 2000;217(1):42–53.
Dogra D, Ahuja S, Kim H-T, Rasouli SJ, Stainier DY, Reischauer S. Opposite effects of Activin type 2 receptor ligands on cardiomyocyte proliferation during development and repair. Nat Commun. 2017;8(1):1–15.
Yang J, Wang J, Zeng Z, Qiao L, Zhuang L, Jiang L, et al. Smad4 is required for the development of cardiac and skeletal muscle in zebrafish. Differentiation. 2016;92(4):161–8.
Qi X, Yang G, Yang L, Lan Y, Weng T, Wang J, et al. Essential role of Smad4 in maintaining cardiomyocyte proliferation during murine embryonic heart development. Dev Biol. 2007;311(1):136–46.
Zhao M, New L, Kravchenko VV, Kato Y, Gram H, Di Padova F, et al. Regulation of the MEF2 family of transcription factors by p38. Mol Cell Biol. 1999;19(1):21–30.
Chen S, Qiong Y, Gardner DG. A role for p38 mitogen-activated protein kinase and c-myc in endothelin-dependent rat aortic smooth muscle cell proliferation. Hypertension. 2006;47(2):252–8.
Balakrishnan S, Sadasivam M, Kannan A, Panneerselvam A, Prahalathan C. Glucose modulates Pax6 expression through the JNK/p38 MAP kinase pathway in pancreatic beta-cells. Life Sci. 2014;109(1):1–7.
Matsumoto-Ida M, Takimoto Y, Aoyama T, Akao M, Takeda T, Kita T. Activation of TGF-β1-TAK1-p38 MAPK pathway in spared cardiomyocytes is involved in left ventricular remodeling after myocardial infarction in rats. Am J Phys Heart Circ Phys. 2006;290(2):H709–H15.
Uosaki H, Magadum A, Seo K, Fukushima H, Takeuchi A, Nakagawa Y, et al. Identification of chemicals inducing cardiomyocyte proliferation in developmental stage–specific manner with pluripotent stem cells. Circ Cardiovasc Genet. 2013;6(6):624–33.
Engel FB, Schebesta M, Duong MT, Lu G, Ren S, Madwed JB, et al. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 2005;19(10):1175–87.
Mebratu Y, Tesfaigzi Y. How ERK1/2 activation controls cell proliferation and cell death: is subcellular localization the answer? Cell Cycle. 2009;8(8):1168–75.
Li P, Cavallero S, Gu Y, Chen TH, Hughes J, Hassan AB, et al. IGF signaling directs ventricular cardiomyocyte proliferation during embryonic heart development. Development. 2011;138(9):1795–805.
Tan L, Bogush N, Naib H, Perry J, Calvert JW, Martin DI, et al. Redox activation of JNK2α2 mediates thyroid hormone-stimulated proliferation of neonatal murine cardiomyocytes. Sci Rep. 2019;9(1):1–15.
Hough C, Radu M, Doré JJ. Tgf-beta induced Erk phosphorylation of smad linker region regulates smad signaling. PLoS One. 2012;7(8):e42513.
Umbarkar P, Singh AP, Gupte M, Verma VK, Galindo CL, Guo Y, et al. Cardiomyocyte SMAD4-dependent TGF-β signaling is essential to maintain adult heart homeostasis. JACC: Basic to Translational Science. 2019;4(1):41–53.
Harvey CD, Ehrhardt AG, Cellurale C, Zhong H, Yasuda R, Davis RJ, et al. A genetically encoded fluorescent sensor of ERK activity. Proc Natl Acad Sci. 2008;105(49):19264–9.
de la Cova C, Townley R, Regot S, Greenwald I. A real-time biosensor for ERK activity reveals signaling dynamics during C. elegans cell fate specification. Dev Cell. 2017;42(5):542–53 e4.
Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332(6028):458–61.
Heallen T, Morikawa Y, Leach J, Tao G, Willerson JT, Johnson RL, et al. Hippo signaling impedes adult heart regeneration. Development. 2013;140(23):4683–90.
Yao M, Wang Y, Zhang P, Chen H, Xu Z, Jiao J, et al. BMP2-SMAD signaling represses the proliferation of embryonic neural stem cells through YAP. J Neurosci. 2014;34(36):12039–48.
Varelas X, Samavarchi-Tehrani P, Narimatsu M, Weiss A, Cockburn K, Larsen BG, et al. The crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-β-SMAD pathway. Dev Cell. 2010;19(6):831–44.
Attisano L, Wrana JL. Signal integration in TGF-β, WNT, and Hippo pathways. F1000prime reports. 2013;5.
Hanna A, Frangogiannis NG. The role of the TGF-beta superfamily in myocardial infarction. Front Cardiovasc Med. 2019;6:140.
Acknowledgments
DWS is supported by National Institutes of Health grant T32GM113846, and JHvB is supported by NIH (HL130072), Regenerative Medicine Minnesota (RMM102516-009), and an Individual Biomedical Research Scholarship from The Hartwell Foundation. Illustrations were provided by L. Sorensen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Daniel Sorensen and Jop van Berlo declare that they have no conflict of interests. This article does not contain any studies with human or animal subjects performed by the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Translational Research in Heart Failure
Rights and permissions
About this article
Cite this article
Sorensen, D.W., van Berlo, J.H. The Role of TGF—β Signaling in Cardiomyocyte Proliferation. Curr Heart Fail Rep 17, 225–233 (2020). https://doi.org/10.1007/s11897-020-00470-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-020-00470-2