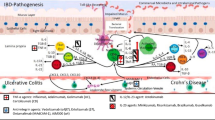
Abstract
Advances (from 2004 to 2006) in the use of conventional agents include the molecular mechanisms of action, which have implications for monitoring (azathioprine and thioguanosine triphosphate) and chemoprevention (mesalamine and peroxisome proliferator activated receptor [PPAR]γ). Advances in biotherapy include new data on monoclonal antibodies (infliximab in ulcerative colitis, adalimumab, certolizumab pegol, fontolizumab, selective anti-adhesion molecules, and others), antisense oligonucleotides, the development of small molecules, and cell-gene therapy (including helminth ova, leukocytapheresis, stem cell transplantation, and probiotic intestinal mucosal delivery systems). However, management of inflammatory bowel disease is about more than drug therapy, dose, and timing. The goals remain induction of remission, limitation of side effects, modification of the pattern of disease, and avoidance of complications. With the cost and complexity of biotherapy, inflammatory bowel disease is emerging as a specific subspecialty.
Similar content being viewed by others
References and Recommended Reading
Bebb JR, Scott BB: Systematic review: How effective are the usual treatments for ulcerative colitis? Aliment Pharmacol Ther 2004, 20:143–149.
Bebb JR, Scott BB: Systematic review: How effective are the usual treatments for Crohn’s disease? Aliment Pharmacol Ther 2004, 20:151–159.
Hanauer SB, Stromberg U: Oral Pentasa in the treatment of active Crohn’s disease: a meta-analysis of double-blind, placebo-controlled trials. Clin Gastroenterol Hepatol 2004, 2:379–388. This paper changed the perception of mesalamine as therapy for mild to moderate ileal Crohn’s disease (results in text).
Feagan BG: 5-ASA therapy for active Crohn’s disease: old friends, old data, and a new conclusion. Clin Gastroenterol Hepatol 2004, 2:376–378.
Hanauer SB: The case for using 5-aminosalicylates in Crohn’s disease: pro. Inflamm Bowel Dis 2005, 11:609–612.
Hanauer SB, Sandborn WJ, Katz S, et al.: Efficacy and safety of mesalazine 4.8g/day (800mg tablet) compared with 2.4g/day (400mg tablet) in treating moderately active ulcerative colitis: ASCEND II study. Gut 2005, 54(Suppl II):A3.
Hanauer SB, Sandborn W, Kornbluth A, et al.: Delayed-release mesalamine 4.8g/day (800mg tablet) versus 2.4g/day (400mg tablet) for treatment of moderately active ulcerative colitis: combined analysis of two randomised, double-blind, controlled trials. Gastroenterology 2005, 128(Suppl 2):A-74.
Kane S, Huo D, Aikens J, Hanauer S: Medication nonadherence and the outcomes of patients with quiescent ulcerative colitis. Am J Med 2003, 114:39–43.
Marteau PR, Probert C, Lindgren S, et al.: Combined oral and enema treatment with Pentasa (mesalazine) is superior to oral therapy alone in patients with extensive mild/moderate active colitis: a randomised, double-blind, placebo controlled study. Gut 2005, 54:960–965. This report confirmed that combined oral and topical therapy, rather than oral mesalamine alone, is more effective for inducing remission for active ulcerative colitis.
Travis SPL: Transatlantic divide: first line therapy for acute colitis. Pract Gastroenterol 2005, 29:66–70.
Rousseaux C, Lefebvre B, Dubuquoy L, et al.: Intestinal antiinflammatory effect of 5-aminosalicylic acid is dependent on peroxisome proliferator-activated receptor-gamma. J Exp Med 2005, 201:1205–1215.
Van Staa TP, Card TR, Leufkens HG, Logan RF: 5-aminosalicylate use and colorectal cancer risk in inflammatorybowel disease: a large epidemiological study. Gut 2005 Jun 30, [Epub ahead of print]. This article provides good evidence for chemopreventive role of 5-ASA. Regular users of mesalamine (6-12 prescriptions before cancer diagnosed) had an OR of 1.13 (0.49-2.59); for 13 to 30 prior prescriptions it was 0.30 (0.11-0.83), and for more than 30 prior prescriptions it was 0.31 (0.11-0.84).
Frieri G, Mariateresa P, Brigida G, et al.: Long-term oral plus topical mesalazine in frequently relapsing ulcerative colitis. Dig Liver Dis 2005, 37:92–96.
Prantera C, Viscido A, Biancone L, et al.: A new oral delivery system for 5-ASA: preliminary clinical findings for MMx. Inflamm Bowel Dis 2005, 11:421–427.
Lichtenstein GR, Cohen RD, Feagan BG, et al.: Safety of in fliximab and other Crohn’s disease therapies: updated Treat registry data with over 10 000 patient-years of follow-up. Gastroenterology 2005, 128(Suppl 2):A-580.
Creed TJ, Dayan CM, Probert CSJ, Hearing SD: Basiliximab for steroid-resistant ulcerative colitis. Gut 2004, 53(Suppl III):A97.
Franchimont D: Overview of the actions of glucocorticoids on the immune response: a good model to characterize new pathways of immunosuppression for new treatment strategies. Ann N Y Acad Sci 2004, 1024:124–137.
Lowenberg M, Verhaar A, vand den Blink B, et al.: Essential role for c-Raf in steroid-insensitive Crohn’s disease. Gastroenterology 2005, 128(Suppl 2):A-505.
Tiede I, Fritz G, Strand S, et al.: CD28-dependent Rac 1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest 2003, 111:1143–1145.
Aberra FN, Lichtenstein GR: Review article: Monitoring of immunomodulators in inflammatory bowel disease. Aliment Pharmacol Ther 2005, 21:307–319.
Neurath MF, Kiesslich R, Tiechgräber U, et al.: Analysis of 6-thioguanosine di-(TGDP) and triphosphate (TGTP) levels as a novel method for clinical monitoring of azathioprine therapy in Crohn’s disease. Gastroenterology 2005, 128(Suppl 2):A-12.
Hanauer SB, Korelitz BI, Rutgeerts P, et al.: Post-operative maintenance of Crohn’s disease remission with 6-mercaptopurine, mesalamine or placebo: a 2 year trial. Gastroenterology 2004, 127:723–729.
Ardizzone S, Maconi G, Sampietro GM, et al.: Azathioprine and mesalamine for prevention of relapse after conservative surgery for Crohn’s disease. Gastroenterology 2004, 127:730–737.
Cosnes J, Nion-Larmurier I, Beaugere L, et al.: Impact of the increasing use of immunosuppressants in Crohn’s disease on the need for intestinal surgery. Gut 2005, 54:237–241.
Dejaco C, Angelberger S, Waldhoer T, et al.: Pregnancy and birth outcome under thiopurine therapy for inflammatory bowel disease. Gastroenterology 2005, 128(Suppl. 2):A-12.
Siegel CA, Sands BE: Review article: Practical management of inflammatory bowel disease patients taking immunomodulators. Aliment Pharmacol Ther 2005, 22:1–16.
Cummings JRF, Herrlinger KR, Travis SP, et al.: Oral methotrexate in ulcerative colitis. Aliment Pharmacol Ther 2005, 21:385–389.
Van Assche G, D’Haens G, Noman M, et al.: Randomized, double-blind comparison of 4mg/kg versus 2mg/kg intravenous cyclosporine in severe ulcerative colitis. Gastroenterology 2003, 125:1025–1031.
Järnerot G, Hertervig E, Friis-Liby I, et al.: In fliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology 2005, 128:1805–11. Small but pivotal trial showing efficacy of IFX for in-patients with severe, active ulcerative colitis refractory to intravenous steroids (results and comparison with ACT trials in text).
Campbell S, Travis SPL, Jewell DP: Ciclosporin use in acute ulcerative colitis: a long-term experience. Eur J Gastroenterol Hepatol 2005, 17:79–84.
Baumgart DC, Pintoffl JP, Sturm A, et al.: Tacrolimus (FK506) rescue therapy is safe and effective in patients with severe and refractory inflammatory bowel disease: a long term follow up. Gastroenterology 2005, 128(Suppl 2):A-198.
Rutgeerts P, Feagan BG, Olson A, et al.: A randomised placebocontrolled trial of infliximab therapy for active ulcerative colitis: Act 1 trial. Gastroenterology 2005, 128(Suppl 2):A-105.
Sandborn WJ, Rachmilewitz D, Hanauer SB, P, et al.: In fliximab induction and maintenance therapy for active ulcerative colitis: the Act 2 trial. Gastroenterology 2005, 128(Suppl 2):A-104.
Targan SR, Hanauer SB, Van Deventer SJ, et al.: A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med 1997, 337:1029–1035.
Hommes DW, Baert F, Van Assche G, et al.: Management of recent onset Crohn’s disease: a controlled randomized trial comparing step-up and top-down therapy. Gastroenterology 2005, in press.
Lémman M, Mary J-Y, Duclos B, et al.: Infliximab as a bridge therapy in steroid-dependent Crohn’s disease patients treated with azathioprine. A randomized, double-blind, placebo-controlled trial. Gastroenterology 2005, in press.
Noman M, Vermeire S, Van Assche G, et al.: The effectiveness of immunosuppression to suppress formation of antibodies to infliximab in Crohn’s disease. Gut 2004, 53(Suppl. VI):A-47.Z.
Ghosh S: Anti-TNF therapy in Crohn’s disease. Novartis Found Symp 2004, 263:193–205, discussion 205–218.
Rampton DS: Preventing TB in patients with Crohn’s disease needing in fliximab or other anti-TNF therapy. Gut 2005, 54:1360–1362.
Shih CE, Bayless TM, Harris ML: Maintenance of longterm response to in fliximab over 1 to 5 years in Crohn’s disease including shortening doing intervals or increasing dosage. Gastroenterology 2004, 126(Suppl 2):A-631.
Williams JB, Cross RK, Thameen D, et al.: Long-term in fliximab maintenance infusion regimens and rates of hospital lisation, surgery and disability in Crohn’s disease patients. Gastroenterology 2005, 128(Suppl 2):A-589.
Rutgeerts P, Feagan BG, Lichtenstein GR, et al.: Comparison of scheduled and episodic treatment strategies of infliximab in Crohn’s disease. Gastroenterology 2004, 126:402–413. Further analysis of ACCENT trial showing that episodic therapy is associated with shorter response, less mucosal healing, and higher rates of hospitalization and abdominal surgery.
Lichtenstein GR, Yan S, Bala M, et al.: In fliximab maintenance treatment reduces hospitalizations, surgeries and procedures in fistulizing Crohn’s disease. Gastroenterology 2005, 128:862–869.
Jewell DP, Satsangi J, Lobo A, et al.: In fliximab use in Crohn’s disease: impact on health care resources in the UK. Eur J Gastroenterol Hepatol 2005, 17:1047–1052.
Nesbitt AM, Henry AJ: High affinity and potency of the pegylated FAB’ fragment CDP870: a direct comparison with other anti-TNF agents. Gut 2004, 53(Suppl VI):A-47.
PRECiSE. UCB announce that CIMZIA TM demonstrates significant positive results in its two pivotal phase III Crohn’s disease trials. Internet press release 26 July 2005. Accessible at: http://ir.ucb-group.com/phoenix.zhtml?c=137495&p=irolnewsArticle& ID=735014&highlight=
MacIntosh DG, Lukas M, Sandborn W, et al.: A randomised, double-blind, placebo-controlled trial of the clinical assessment of adalimumab safety and efficacy studied as an induction therapy in Crohn’s disease. Gut 2004, 53(Suppl VI):A-47.
Sandborn W, Hanauer S, Lukas M, et al.: Induction and maintenance of clinical remission and response in subjects with Crohn’s disease treated during a 6-month open-label period with fully human anti-TNF alpha monoclonal antibody adalimumab (Humira). Gastroenterology 2005, 128(Suppl 2):A-111.
Papadakis KA, Shaye OA, Vasiliauskas EA, et al.: Safety and efficacy of adalimumab (D2E7) in Crohn’s disease patients with an attenuated response to in fliximab. Am J Gastroenterol 2005, 100:75–79.
Rutgeerts P, Reinisch W, Colombel J-F, et al.: Preliminary results of a phase I/II study of HuZAF, an anti-IFNg monoclonal antibody in patients with moderate to severe active Crohn’s disease. Gastroenterology 2002, 122 Suppl 4:A-61.
Van Assche G, Pearce T: Fontolizumab (Huzaf), a humanized anti-IFN-gamma antibody, has clinical activity and excellent tolerability in moderate to severe Crohn’s disease. Gut 2004, 53(Suppl VI):A48.
De Villiers W, Katz S, Salzberg BA, et al.: Chronic dosing of fontolizumab (Huzaf), a humanised anti-IFN-gamma antibody in patients with moderate to severe Crohn’s disease. Gastroenterology 2005, 128(Suppl 2):A-111.
Targan SR, Salzberg BA, Mayer L, et al.: A phase I-II study: multiple dose levels of visilizumab are well tolerated and produce rapid and sustained improvement in ulcerative colitis patients refractory to treatment with intravenous steroids (IVSR-UC). Gastroenterology 2005, 128(Suppl 2):A-75.
Hommes DW, Plevy S, Salzberg BA, et al.: Epstein-Barr virus (EBV) replication in severe active, steroid-resistant ulcerative colitis patients treated with visilizumab, an anti-CD3 antibody. Gastroenterology 2005, 128(Suppl. 2):A-75.
Mannon PJ, Fuss I, Mayer L, et al.: Anti-interleukin-12 antibody for active Crohn’s disease. N Engl J Med 2004, 351:2069–2079.
Ito H, Takazoe M, Fukuda Y, et al.: A pilot randomised trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn’s disease. Gastroenterology 2004, 126:989–996.
Feagan BG, Greenberg GR, Wild G, et al.: Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med 2005, 352:2499–2507.
Rutgeerts P, Enns R, Colombel JF, et al.: 6-month steroid-sparing results of natalizumab in a controlled study of patients with Crohn’s disease. Gut 2004, 53(Suppl. VI):A-48.
Van Assche G, Van Ranst M, Sciot R, et al.: Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med 2005, 353:362–368.
Chey WY, Volfova M, Konecny M, et al.: Two phase 3 studies of alicaforsen (ISIS2302), an anti-sense oligonulceotide to human ICAM-1, in the treatment of moderate to severe Crohn’s disease. Gastroenterology 2005, 128(Suppl. 2):A-112.
Van Deventer SJ, Volfova M, Flisiak R, et al.: A phase 2 doseranging, double blind, placebo-controlled study of alicaforsen enema in subjects with acute exacerbation of mild to moderate left-sided ulcerative colitis. Gastroenterology 2005, 128(Suppl. 2):A-74.
Miner PB, Nichols T, Schwartz H, et al.: A phase 2 trial to assess the safety and efficacy of two dose formulations of alicaforsen enema compared with mesalamine enema for acute ulcerative colitis. Gastroenterology 2005, 128(Suppl. 2):A-74.
Braat H, Rottiers P, Huyghebaert N, et al.: Interleukin-10 producing Lactococcus lactis for the treatment of Crohn’s disease. Gastroenterology 2005, 128(Suppl. 2):A-104.
Herrlinger KR, Witthoeft T, Raedler A, et al.: Randomised, doubleblind, double-dummy, controlled trial of subcutaneous recombinant human interleukin-11 vs prednisolone in active Crohn’s disease. Gut 2004, 53(Suppl. VI):A-49.
Mahmood A, Melley L, Fitzgerald A, et al.: Phase I/II trial of trefoil factor family 3 (TFF-3) enema therapy with oral mesalazine for mild to moderate left-sided colitis. Gastroenterology 2005, 128(Suppl. 2):A-581.
Korzenik JR, Dieckgraefe B, Valentine JF, et al.: Sargramostim for active Crohn’s disease. N Engl J Med 2005, 352:2193–2201.
Valentine J, Stone C, Korzenik J, et al.: Repeated cycles of sargramostim for active Crohn’s disease: update from an open label trial (n.o.v.e.l. 5). Gastroenterology 2005, 128(Suppl. 2):A-111.
Buchman AL, Katz S, Barish C, et al.: Semapimod treatment of Crohn’s disease. Gastroenterology 2004, 126 Suppl 2:A-464.
Travis SPL, Yap LM, Hawkey CJ, et al.: RDP58—a novel and potentially effective oral therapy for ulcerative colitis (UC): results of parallel prospective, multicenter, blinded, placebocontrolled trials. Inflamm Bowel Dis 2005, in press.
Hanauer SB, Miner PB, Keshavarzian A, et al.: Randomized, double-blind, placebo-controlled, parallel-arm, safety and efficacy trial of once-daily, oral OPC-6535 in the treatment of active ulcerative colitis. Gastroenterology 2004, 126Suppl 2:A-112.
Schreiber SW, Forbes A, Feagan BG, et al.: Randomized, multicentre, double blind, placebo-controlled trial of once daily, oral OPC-6535 in the treatment of active ulcerative colitis: use of the disease activity index (DAI) to define study population and treatment response. Gut 2004, 53(Suppl. VI):A-225.
Rieder F, Siegmund B, Lehr HA, et al.: The novel specific type-4 phosphodiesterase inhibitor roflumilast mitigates experimental colitis in mice. Gastroenterology 2005, 128(Suppl. 2):A-203.
Summers RW, Elliott DE, Urban JF Jr, et al.: Trichuris suis therapy in Crohn’s disease. Gut 2005, 54:87–90.
Summers RW, Elliott DE, Urban JF Jr, et al.: Trichuris suis therapy for active ulcerative colitis: a randomised controlled trial. Gastroenterology 2005, 128:825–32. Proof of concept of a novel approach to therapy for active ulcerative colitis (and Crohn’s disease [73]).
O’Neil J, Speare R, Melrose W, et al.: A pilot study establishing Necator americanus in Crohn’s disease patients and normal controls. Gastroenterology 2005, 128: abstract.
Irving PM, Rampton DS: Leucocytapheresis for ulcerative colitis. Dig Liver Dis 2004, 36:799–802.
Barkholt L, Lofberg R: Resetting the immune system in refractory Crohn’s disease: autologous haemopoetic stem cell transplantation the way forward? Gastroenterology 2005, 128:786–789.
Shanahan F: Physiological basis for novel drug therapies used to treat inflammatory bowel diseases I. Pathophysiological basis and prospects for probiotic therapy in inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol 2005, 288:G417-G421. Useful review of the science in a complex field characterized more by confusion in complementary medicine than precision in practice.
Cummings JH, Kong SC: Probiotics, prebiotics and antibiotics in inflammatory bowel disease. Novartis Found Symp 2004, 11:75–90.
Gionchetti P, Lammers KM, Rizzello F, Campieri M: Probiotics and barrier function in colitis. Gut 2005, 54:898–900.
Stremmel W, Merle U, Zahn A, et al.: Retarded release phosphatidyl choline benefits patients with chronic active ulcerative colitis. Gut 2005, 54:966–971.
Su C, Lichtenstein GR, Krok K, et al.: A meta-analysis of the placebo rates of remission and response in clinical trials of active Crohn’s disease. Gastroenterology 2004, 126:1257–1269.
Su C, Lewis JD, Goldberg B, et al.: Factors that influence placebo remission rates in clinical trials of active ulcerative colitis: a meta-analysis. Gastroenterology 2005, 128(Suppl. 2):A-326.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Travis, S. Advances in therapeutic approaches to ulcerative colitis and crohn’s disease. Curr Gastroenterol Rep 7, 475–484 (2005). https://doi.org/10.1007/s11894-005-0079-9
Issue Date:
DOI: https://doi.org/10.1007/s11894-005-0079-9