Abstract
Purpose of review
Grafted beta cells are lost because of recurrence of T1D and/or allograft rejection, two conditions diagnosed with pancreas graft biopsy, which is invasive and impossible in case of islet transplantation. This review synthetizes the current pathophysiological knowledge and discusses the interest of available immune biomarkers.
Recent findings
Despite the central role of auto-(recurrence of T1D) and allo-(T-cell mediated rejection) immune cellular responses, the latter are not directly monitored in routine. In striking contrast, there have been undisputable progresses in monitoring of auto and alloantibodies.
Summary
Except for pancreas recipients in whom anti-donor HLA antibodies can be directly responsible for antibody-mediated rejection, autoantibodies (and alloantibodies in islet recipients) have no direct pathogenic effect. However, their fluctuation offers a surrogate marker for the activation status of T cells (because antibody generation depends on T cells). This illustrates the necessity to understand the pathophysiology when interpreting a biomarker and selecting the appropriate treatment.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Wojtusciszyn A, Branchereau J, Esposito L, Badet L, Buron F, Chetboun M, et al. Indications for islet or pancreatic transplantation: statement of the TREPID working group on behalf of the Societe francophone du diabete (SFD), Societe francaise d'endocrinologie (SFE), Societe francophone de transplantation (SFT) and Societe francaise de nephrologie - dialyse - transplantation (SFNDT). Diabetes Metab. 2019;45(3):224–37. https://doi.org/10.1016/j.diabet.2018.07.006This review explains the benefit / risk ratios of each beta cell replacement therapy and proposes a decision tree for transplantation indications.
Azmi S, Jeziorska M, Ferdousi M, Petropoulos IN, Ponirakis G, Marshall A, et al. Early nerve fibre regeneration in individuals with type 1 diabetes after simultaneous pancreas and kidney transplantation. Diabetologia. 2019;62(8):1478–87. https://doi.org/10.1007/s00125-019-4897-y.
Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med. 1998;339(2):69–75. https://doi.org/10.1056/NEJM199807093390202.
Fiorina P, Gremizzi C, Maffi P, Caldara R, Tavano D, Monti L, et al. Islet transplantation is associated with an improvement of cardiovascular function in type 1 diabetic kidney transplant patients. Diabetes Care. 2005;28(6):1358–65. https://doi.org/10.2337/diacare.28.6.1358.
Giannarelli R, Coppelli A, Sartini MS, Del Chiaro M, Vistoli F, Rizzo G, et al. Pancreas transplant alone has beneficial effects on retinopathy in type 1 diabetic patients. Diabetologia. 2006;49(12):2977–82. https://doi.org/10.1007/s00125-006-0463-5.
La Rocca E, Fiorina P, di Carlo V, Astorri E, Rossetti C, Lucignani G, et al. Cardiovascular outcomes after kidney-pancreas and kidney-alone transplantation. Kidney Int. 2001;60(5):1964–71. https://doi.org/10.1046/j.1523-1755.2001.00008.x.
Thompson DM, Meloche M, Ao Z, Paty B, Keown P, Shapiro RJ, et al. Reduced progression of diabetic microvascular complications with islet cell transplantation compared with intensive medical therapy. Transplantation. 2011;91(3):373–8. https://doi.org/10.1097/TP.0b013e31820437f3.
Vantyghem MC, Quintin D, Caiazzo R, Leroy C, Raverdy V, Cassim F, et al. Improvement of electrophysiological neuropathy after islet transplantation for type 1 diabetes: a 5-year prospective study. Diabetes Care. 2014;37(6):e141–2. https://doi.org/10.2337/dc14-0320.
Fiorina P, Folli F, Bertuzzi F, Maffi P, Finzi G, Venturini M, et al. Long-term beneficial effect of islet transplantation on diabetic macro-/microangiopathy in type 1 diabetic kidney-transplanted patients. Diabetes Care. 2003;26(4):1129–36. https://doi.org/10.2337/diacare.26.4.1129.
Mohan P, Safi K, Little DM, Donohoe J, Conlon P, Walshe JJ, et al. Improved patient survival in recipients of simultaneous pancreas-kidney transplant compared with kidney transplant alone in patients with type 1 diabetes mellitus and end-stage renal disease. Br J Surg. 2003;90(9):1137–41. https://doi.org/10.1002/bjs.4208.
Benhamou PY, Milliat-Guittard L, Wojtusciszyn A, Kessler L, Toso C, Baertschiger R, et al. Quality of life after islet transplantation: data from the GRAGIL 1 and 2 trials. Diabet Med. 2009;26(6):617–21. https://doi.org/10.1111/j.1464-5491.2009.02731.x.
Sureshkumar KK, Patel BM, Markatos A, Nghiem DD, Marcus RJ. Quality of life after organ transplantation in type 1 diabetics with end-stage renal disease. Clin Transpl. 2006;20(1):19–25. https://doi.org/10.1111/j.1399-0012.2005.00433.x.
Gruessner AC, Gruessner RW. Pancreas Transplantation of US and Non-US Cases from 2005 to 2014 as Reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR). Rev Diabet Stud. 2016;13(1):35–58 10.1900/RDS.2016.13.e2016002. 10.1900/RDS.2016.13.35.
Vantyghem MC, Chetboun M, Gmyr V, Jannin A, Espiard S, Le Mapihan K, et al. Ten-year outcome of islet alone or islet after kidney transplantation in type 1 diabetes: a prospective parallel-arm cohort study. Diabetes Care. 2019;42(11):2042–9. https://doi.org/10.2337/dc19-0401.
Rickels MR, Stock PG, de Koning EJP, Piemonti L, Pratschke J, Alejandro R, et al. Defining Outcomes for beta-cell Replacement Therapy in the Treatment of Diabetes: A Consensus Report on the Igls Criteria From the IPITA/EPITA Opinion Leaders Workshop. Transplantation. 2018;102(9):1479–86. https://doi.org/10.1097/TP.0000000000002158This paper is the first consensus report on definitions of function and failure of different forms of beta cell replacement therapy, on behalf of the International Pancreas and Islet Transplant Association and the European Pancreas and Islet Transplantation Association.
Drachenberg CB, Odorico J, Demetris AJ, Arend L, Bajema IM, Bruijn JA, et al. Banff schema for grading pancreas allograft rejection: working proposal by a multi-disciplinary international consensus panel. Am J Transplant. 2008;8(6):1237–49. https://doi.org/10.1111/j.1600-6143.2008.02212.x.
Lehmann-Werman R, Neiman D, Zemmour H, Moss J, Magenheim J, Vaknin-Dembinsky A, et al. Identification of tissue-specific cell death using methylation patterns of circulating DNA. Proc Natl Acad Sci U S A. 2016;113(13):E1826–34. https://doi.org/10.1073/pnas.1519286113.
Marchand L, Jalabert A, Meugnier E, Van den Hende K, Fabien N, Nicolino M, et al. miRNA-375 a Sensor of Glucotoxicity Is Altered in the Serum of Children with Newly Diagnosed Type 1 Diabetes. J Diabetes Res. 2016;2016:1869082. https://doi.org/10.1155/2016/1869082.
Chang CA, Haque WZ, Yoshimatsu G, Balajii PS, Lawrence MC, Naziruddin B. Monitoring of beta cell replacement outcomes. Panminerva Med. 2016;58(1):59–71.
Sutherland DE, Goetz FC, Sibley RK. Recurrence of disease in pancreas transplants. Diabetes. 1989;38(Suppl 1):85–7. https://doi.org/10.2337/diab.38.1.s85.
Petruzzo P, Andreelli F, McGregor B, Lefrancois N, Dawahra M, Feitosa LC, et al. Evidence of recurrent type I diabetes following HLA-mismatched pancreas transplantation. Diabetes Metab. 2000;26(3):215–8.
Tyden G, Reinholt FP, Sundkvist G, Bolinder J. Recurrence of autoimmune diabetes mellitus in recipients of cadaveric pancreatic grafts. N Engl J Med. 1996;335(12):860–3. https://doi.org/10.1056/NEJM199609193351205.
Vendrame F, Hopfner YY, Diamantopoulos S, Virdi SK, Allende G, Snowhite IV, et al. Risk Factors for Type 1 Diabetes Recurrence in Immunosuppressed Recipients of Simultaneous Pancreas-Kidney Transplants. Am J Transplant. 2016;16(1):235–45. https://doi.org/10.1111/ajt.13426This study is the first assessing the incidence and risk factors of autoimmune recurrence (biopsy-confirmed in 88% patients) in a cohort of 223 simultaneous pancreas – kidney recipients.
Stegall MD, Lafferty KJ, Kam I, Gill RG. Evidence of recurrent autoimmunity in human allogeneic islet transplantation. Transplantation. 1996;61(8):1272–4. https://doi.org/10.1097/00007890-199604270-00027.
Worcester Human Islet Transplantation G, Sharma V, Andersen D, Thompson M, Woda BA, Stoff JS, et al. Autoimmunity after islet-cell allotransplantation. N Engl J Med. 2006;355(13):1397–9. https://doi.org/10.1056/NEJMc061530.
Robertson CC, Rich SS. Genetics of type 1 diabetes. Curr Opin Genet Dev. 2018;50:7–16. https://doi.org/10.1016/j.gde.2018.01.006.
Ilonen J, Lempainen J, Veijola R. The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat Rev Endocrinol. 2019;15(11):635–50. https://doi.org/10.1038/s41574-019-0254-yThis review explains the pathogenesis of type 1 diabetes mellitus, detailing genetic factors (and their role in the generation of autoimmune response), environmental factors (such as microbiota composition, microbial infections and nutrition) and their role in the evolution of the incidence of type 1 diabetes mellitus in the last half of the twentieth century.
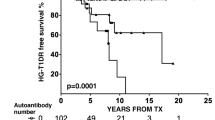
Piemonti L, Everly MJ, Maffi P, Scavini M, Poli F, Nano R, et al. Alloantibody and autoantibody monitoring predicts islet transplantation outcome in human type 1 diabetes. Diabetes. 2013;62(5):1656–64. https://doi.org/10.2337/db12-1258.
Mallone R, Roep BO. Biomarkers for immune intervention trials in type 1 diabetes. Clin Immunol. 2013;149(3):286–96. https://doi.org/10.1016/j.clim.2013.02.009.
Pugliese A. Autoreactive T cells in type 1 diabetes. J Clin Invest. 2017;127(8):2881–91. https://doi.org/10.1172/JCI94549.
Roep BO, Kracht MJ, van Lummel M, Zaldumbide A. A roadmap of the generation of neoantigens as targets of the immune system in type 1 diabetes. Curr Opin Immunol. 2016;43:67–73. https://doi.org/10.1016/j.coi.2016.09.007.
Thomaidou S, Zaldumbide A, Roep BO. Islet stress, degradation and autoimmunity. Diabetes Obes Metab. 2018;20 Suppl 2:88–94. https://doi.org/10.1111/dom.13387.
Monti P, Vignali D, Piemonti L. Monitoring inflammation, humoral and cell-mediated immunity in pancreas and islet transplants. Curr Diabetes Rev. 2015;11(3):135–43. https://doi.org/10.2174/1573399811666150317125820.
Szempruch KR, Banerjee O, McCall RC, Desai CS. Use of anti-inflammatory agents in clinical islet cell transplants: a qualitative systematic analysis. Islets. 2019;11(3):65–75. https://doi.org/10.1080/19382014.2019.1601543.
Chen CC, Pouliquen E, Broisat A, Andreata F, Racape M, Bruneval P, et al. Endothelial chimerism and vascular sequestration protect pancreatic islet grafts from antibody-mediated rejection. J Clin Invest. 2018;128(1):219–32. https://doi.org/10.1172/JCI93542This translational study demonstrates the resistance of islet allograft to humoral rejection, which is due to endothelial chimerism (endothelial cells being mainly from recipient origin) and vascular sequestration of donor-specific anti-HLA antibodies. This finding may have important implications outside the field of beta cell replacement.
Bloem SJ, Roep BO. The elusive role of B lymphocytes and islet autoantibodies in (human) type 1 diabetes. Diabetologia. 2017;60(7):1185–9. https://doi.org/10.1007/s00125-017-4284-5.
Lampasona V, Pittman DL, Williams AJ, Achenbach P, Schlosser M, Akolkar B, et al. Islet Autoantibody Standardization Program 2018 Workshop: Interlaboratory Comparison of Glutamic Acid Decarboxylase Autoantibody Assay Performance. Clin Chem. 2019;65(9):1141–52. https://doi.org/10.1373/clinchem.2019.304196.
Mathieu C, Lahesmaa R, Bonifacio E, Achenbach P, Tree T. Immunological biomarkers for the development and progression of type 1 diabetes. Diabetologia. 2018;61(11):2252–8. https://doi.org/10.1007/s00125-018-4726-8This is a recent review on immunological biomarkers for the follow-up of type 1 diabetes mellitus, including genetic biomarkers, autoantibodies, T cell biomarkers, emerging biomarkers (omics) and the role of longitudinal and integrated biomarker studies.
Assalino M, Genevay M, Morel P, Demuylder-Mischler S, Toso C, Berney T. Recurrence of type 1 diabetes after simultaneous pancreas-kidney transplantation in the absence of GAD and IA-2 autoantibodies. Am J Transplant. 2012;12(2):492–5. https://doi.org/10.1111/j.1600-6143.2011.03844.x.
Martins LS, Henriques AC, Fonseca IM, Rodrigues AS, Oliverira JC, Dores JM, et al. Pancreatic autoantibodies after pancreas-kidney transplantation - do they matter? Clin Transpl. 2014;28(4):462–9. https://doi.org/10.1111/ctr.12337.
Occhipinti M, Lampasona V, Vistoli F, Bazzigaluppi E, Scavini M, Boggi U, et al. Zinc transporter 8 autoantibodies increase the predictive value of islet autoantibodies for function loss of technically successful solitary pancreas transplant. Transplantation. 2011;92(6):674–7. https://doi.org/10.1097/TP.0b013e31822ae65f.
Bosi E, Braghi S, Maffi P, Scirpoli M, Bertuzzi F, Pozza G, et al. Autoantibody response to islet transplantation in type 1 diabetes. Diabetes. 2001;50(11):2464–71. https://doi.org/10.2337/diabetes.50.11.2464.
Ahmed S, Cerosaletti K, James E, Long SA, Mannering S, Speake C, et al. Standardizing T-Cell Biomarkers in Type 1 Diabetes: Challenges and Recent Advances. Diabetes. 2019;68(7):1366–79. https://doi.org/10.2337/db19-0119This paper describes T cell biomarkers (antigen-specific or antigen-agnostic) used in type 1 diabetes mellitus, their place in the follow-up of response to therapy and the challenges to develop effective T cell biomarkers in type 1 diabetes.
Terrazzano G, Bruzzaniti S, Rubino V, Santopaolo M, Palatucci AT, Giovazzino A, et al. T1D progression is associated with loss of CD3(+)CD56(+) regulatory T cells that control CD8(+) T cell effector functions. Nat Metab. 2020;2(2):142–52. https://doi.org/10.1038/s42255-020-0173-1.
Jacobsen LM, Newby BN, Perry DJ, Posgai AL, Haller MJ, Brusko TM. Immune Mechanisms and Pathways Targeted in Type 1 Diabetes. Curr Diab Rep. 2018;18(10):90. https://doi.org/10.1007/s11892-018-1066-5.
Laughlin E, Burke G, Pugliese A, Falk B, Nepom G. Recurrence of autoreactive antigen-specific CD4+ T cells in autoimmune diabetes after pancreas transplantation. Clin Immunol. 2008;128(1):23–30. https://doi.org/10.1016/j.clim.2008.03.459.
Velthuis JH, Unger WW, van der Slik AR, Duinkerken G, Engelse M, Schaapherder AF, et al. Accumulation of autoreactive effector T cells and allo-specific regulatory T cells in the pancreas allograft of a type 1 diabetic recipient. Diabetologia. 2009;52(3):494–503. https://doi.org/10.1007/s00125-008-1237-z.
Vendrame F, Pileggi A, Laughlin E, Allende G, Martin-Pagola A, Molano RD, et al. Recurrence of type 1 diabetes after simultaneous pancreas-kidney transplantation, despite immunosuppression, is associated with autoantibodies and pathogenic autoreactive CD4 T-cells. Diabetes. 2010;59(4):947–57. https://doi.org/10.2337/db09-0498.
Pugliese A, Reijonen HK, Nepom J, Burke GW 3rd. Recurrence of autoimmunity in pancreas transplant patients: research update. Diabetes Manag (Lond). 2011;1(2):229–38. https://doi.org/10.2217/dmt.10.21.
Burke GW 3rd, Vendrame F, Virdi SK, Ciancio G, Chen L, Ruiz P, et al. Lessons from pancreas transplantation in type 1 diabetes: recurrence of islet autoimmunity. Curr Diab Rep. 2015;15(12):121. https://doi.org/10.1007/s11892-015-0691-5.
Pinkse GG, Tysma OH, Bergen CA, Kester MG, Ossendorp F, van Veelen PA, et al. Autoreactive CD8 T cells associated with beta cell destruction in type 1 diabetes. Proc Natl Acad Sci U S A. 2005;102(51):18425–30. https://doi.org/10.1073/pnas.0508621102.
Huurman VA, Hilbrands R, Pinkse GG, Gillard P, Duinkerken G, van de Linde P, et al. Cellular islet autoimmunity associates with clinical outcome of islet cell transplantation. PLoS One. 2008;3(6):e2435. https://doi.org/10.1371/journal.pone.0002435.
Chujo D, Foucat E, Takita M, Itoh T, Sugimoto K, Shimoda M, et al. Emergence of a broad repertoire of GAD65-specific T-cells in type 1 diabetes patients with graft dysfunction after allogeneic islet transplantation. Cell Transplant. 2012;21(12):2783–95. https://doi.org/10.3727/096368912X654993.
Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J Immunol. 2001;166(2):973–81. https://doi.org/10.4049/jimmunol.166.2.973.
Halloran PF, Chang J, Famulski K, Hidalgo LG, Salazar ID, Merino Lopez M, et al. Disappearance of T cell-mediated rejection despite continued antibody-mediated rejection in late kidney transplant recipients. J Am Soc Nephrol. 2015;26(7):1711–20. https://doi.org/10.1681/ASN.2014060588.
Chen CC, Koenig A, Saison C, Dahdal S, Rigault G, Barba T, et al. CD4+ T cell help is mandatory for naive and memory donor-specific antibody responses: impact of therapeutic immunosuppression. Front Immunol. 2018;9:275. https://doi.org/10.3389/fimmu.2018.00275This paper demonstrates that CD4+ T cell help is mandatory for both naive and memory DSA responses after transplantation and that recipient’s CD4+T cells are not adequately blocked by current maintenance immunosuppressive drugs.
Thaunat O, Koenig A, Leibler C, Grimbert P. Effect of immunosuppressive drugs on humoral allosensitization after kidney transplant. J Am Soc Nephrol. 2016;27(7):1890–900. https://doi.org/10.1681/ASN.2015070781.
Dahdal S, Saison C, Valette M, Bachy E, Pallet N, Lina B, et al. Residual activatability of circulating Tfh17 predicts humoral response to thymodependent antigens in patients on therapeutic immunosuppression. Front Immunol. 2018;9:3178. https://doi.org/10.3389/fimmu.2018.03178.
Pouliquen E, Baltzinger P, Lemle A, Chen CC, Parissiadis A, Borot S, et al. Anti-donor HLA antibody response after pancreatic islet grafting: characteristics, risk factors, and impact on graft function. Am J Transplant. 2017;17(2):462–73. https://doi.org/10.1111/ajt.13936This article assesses de novo donor-specific anti-HLA antibodies (DSA) responses in a cohort of 42 islet graft recipients. DSA do not negatively impact islet graft survival.
Campbell PM, Salam A, Ryan EA, Senior P, Paty BW, Bigam D, et al. Pretransplant HLA antibodies are associated with reduced graft survival after clinical islet transplantation. Am J Transplant. 2007;7(5):1242–8. https://doi.org/10.1111/j.1600-6143.2007.01777.x.
van Kampen CA, van de Linde P, Duinkerken G, van Schip JJ, Roelen DL, Keymeulen B, et al. Alloreactivity against repeated HLA mismatches of sequential islet grafts transplanted in non-uremic type 1 diabetes patients. Transplantation. 2005;80(1):118–26. https://doi.org/10.1097/01.tp.0000164143.22287.e3.
Lim WH, Wong G, Heidt S, Claas FHJ. Novel aspects of epitope matching and practical application in kidney transplantation. Kidney Int. 2018;93(2):314–24. https://doi.org/10.1016/j.kint.2017.08.008.
Duquesnoy RJ. A structurally based approach to determine HLA compatibility at the humoral immune level. Hum Immunol. 2006;67(11):847–62. https://doi.org/10.1016/j.humimm.2006.08.001.
Wiebe C, Rush DN, Nevins TE, Birk PE, Blydt-Hansen T, Gibson IW, et al. Class II Eplet mismatch modulates tacrolimus trough levels required to prevent donor-specific antibody development. J Am Soc Nephrol. 2017;28(11):3353–62. https://doi.org/10.1681/ASN.2017030287.
Chaigne B, Geneugelijk K, Bedat B, Ahmed MA, Honger G, De Seigneux S, et al. Immunogenicity of anti-HLA antibodies in pancreas and islet transplantation. Cell Transplant. 2016;25(11):2041–50. https://doi.org/10.3727/096368916X691673.
Reindl-Schwaighofer R, Heinzel A, Kainz A, van Setten J, Jelencsics K, Hu K, et al. Contribution of non-HLA incompatibility between donor and recipient to kidney allograft survival: genome-wide analysis in a prospective cohort. Lancet. 2019;393(10174):910–7. https://doi.org/10.1016/S0140-6736(18)32473-5.
Reindl-Schwaighofer R, Heinzel A, Signorini L, Thaunat O, Oberbauer R. Mechanisms underlying human genetic diversity: consequence for antigraft antibody responses. Transpl Int. 2018;31(3):239–50. https://doi.org/10.1111/tri.13059.
Tait BD. Detection of HLA antibodies in organ transplant recipients - triumphs and challenges of the solid phase bead assay. Front Immunol. 2016;7:570. https://doi.org/10.3389/fimmu.2016.00570.
Cantarovich D, De Amicis S, Akl A, Devys A, Vistoli F, Karam G, et al. Posttransplant donor-specific anti-HLA antibodies negatively impact pancreas transplantation outcome. Am J Transplant. 2011;11(12):2737–46. https://doi.org/10.1111/j.1600-6143.2011.03729.x.
Malheiro J, Martins LS, Tafulo S, Dias L, Fonseca I, Beirao I, et al. Impact of de novo donor-specific anti-HLA antibodies on grafts outcomes in simultaneous pancreas-kidney transplantation. Transpl Int. 2016;29(2):173–83. https://doi.org/10.1111/tri.12687.
Mittal S, Page SL, Friend PJ, Sharples EJ, Fuggle SV. De novo donor-specific HLA antibodies: biomarkers of pancreas transplant failure. Am J Transplant. 2014;14(7):1664–71. https://doi.org/10.1111/ajt.12750.
Parajuli S, Alagusundaramoorthy S, Aziz F, Garg N, Redfield RR, Sollinger H, et al. Outcomes of Pancreas Transplant Recipients With De Novo Donor-specific Antibodies. Transplantation. 2019;103(2):435–40. https://doi.org/10.1097/TP.0000000000002339This article assesses de novo donor-specific anti-HLA antibodies (DSA) responses in a cohort of 541 pancreas transplant recipients. De novo DSA are associated with increased rates of rejection and graft failure.
Mujtaba MA, Fridell JA, Higgins N, Sharfuddin AA, Yaqub MS, Kandula P, et al. Early findings of prospective anti-HLA donor specific antibodies monitoring study in pancreas transplantation: Indiana University Health Experience. Clin Transpl. 2012;26(5):E492–9. https://doi.org/10.1111/ctr.12005.
Schinstock CA, Cosio F, Cheungpasitporn W, Dadhania DM, Everly MJ, Samaniego-Picota MD, et al. The value of protocol biopsies to identify patients with de novo donor-specific antibody at high risk for allograft loss. Am J Transplant. 2017;17(6):1574–84. https://doi.org/10.1111/ajt.14161.
Rabant M, Amrouche L, Lebreton X, Aulagnon F, Benon A, Sauvaget V, et al. Urinary C-X-C motif chemokine 10 independently improves the noninvasive diagnosis of antibody-mediated kidney allograft rejection. J Am Soc Nephrol. 2015;26(11):2840–51. https://doi.org/10.1681/ASN.2014080797.
Brooks AM, Carter V, Liew A, Marshall H, Aldibbiat A, Sheerin NS, et al. De novo donor-specific HLA antibodies are associated with rapid loss of graft function following islet transplantation in type 1 diabetes. Am J Transplant. 2015;15(12):3239–46. https://doi.org/10.1111/ajt.13407.
Campbell PM, Senior PA, Salam A, Labranche K, Bigam DL, Kneteman NM, et al. High risk of sensitization after failed islet transplantation. Am J Transplant. 2007;7(10):2311–7. https://doi.org/10.1111/j.1600-6143.2007.01923.x.
Mohanakumar T, Narayanan K, Desai N, Ramachandran S, Shenoy S, Jendrisak M, et al. A significant role for histocompatibility in human islet transplantation. Transplantation. 2006;82(2):180–7. https://doi.org/10.1097/01.tp.0000226161.82581.b2.
Rickels MR, Kearns J, Markmann E, Palanjian M, Markmann JF, Naji A, et al. HLA sensitization in islet transplantation. Clin Transpl. 2006;34:413–20.
Cardani R, Pileggi A, Ricordi C, Gomez C, Baidal DA, Ponte GG, et al. Allosensitization of islet allograft recipients. Transplantation. 2007;84(11):1413–27. https://doi.org/10.1097/01.tp.0000290388.70019.6e.
Uva PD, Papadimitriou JC, Drachenberg CB, Toniolo MF, Quevedo A, Dotta AC, et al. Graft dysfunction in simultaneous pancreas kidney transplantation (SPK): results of concurrent kidney and pancreas allograft biopsies. Am J Transplant. 2019;19(2):466–74. https://doi.org/10.1111/ajt.15012.
Delaune V, Toso C, Benhamou PY, Wojtusciszyn A, Kessler L, Slits F, et al. Alloimmune monitoring after islet transplantation: a prospective multicenter assessment of 25 recipients. Cell Transplant. 2016;25(12):2259–68. https://doi.org/10.3727/096368916X692023.
Huurman VA, van der Torren CR, Gillard P, Hilbrands R, van der Meer-Prins EP, Duinkerken G, et al. Immune responses against islet allografts during tapering of immunosuppression—a pilot study in 5 subjects. Clin Exp Immunol. 2012;169(2):190–8. https://doi.org/10.1111/j.1365-2249.2012.04605.x.
Acknowledgements
The authors wish to thank Clemence Thaunat for her help in the design of the figure. OT has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (SC1-BHC-07-2019 - VANGUARD).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Immunology, Transplantation, and Regenerative Medicine
Rights and permissions
About this article
Cite this article
Buron, F., Reffet, S., Badet, L. et al. Immunological Monitoring in Beta Cell Replacement: Towards a Pathophysiology-Guided Implementation of Biomarkers. Curr Diab Rep 21, 19 (2021). https://doi.org/10.1007/s11892-021-01386-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s11892-021-01386-4