Abstract
Purpose of Review
The DEVOTE study compared the cardiovascular safety of two basal insulins, degludec, and glargine U100 in patients with type 2 diabetes (T2D) at high risk for cardiovascular disease (CVD). In this review, we summarize the results of DEVOTE and provide a clinical perspective.
Recent Findings
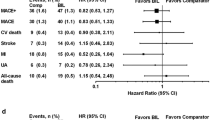
DEVOTE was a phase 3b, multicenter, international, treat-to-target, double-blind, event-driven trial. Patients with T2D > 50 years of age with prior CVD or > 60 years of age with CVD risk factors were randomly assigned to receive either degludec (n = 3818) or insulin glargine U100 (n = 3819) and were followed until at least 633 positively adjudicated major adverse cardiovascular events (MACE; cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke) accrued. At baseline, the mean age of the subjects was 65.0 years, the mean duration of diabetes was 16.4 years, and the mean HbA1c was 8.4 ± 1.7%. After a median follow-up of 2 years, HbA1c had decreased to 7.5 ± 1.2% in each group. Degludec was non-inferior to insulin glargine U100 with respect to the primary MACE outcome (hazard ratio 0.91; 95% CI 0.78–1.06). Significantly, lower rates of severe hypoglycemia and nocturnal severe hypoglycemia were observed with degludec compared to glargine U100 (rate ratios of 0.60; 95% CI 0.48–0.76 and 0.47; 95% CI 0.31 to 0.73, respectively).
Summary
DEVOTE demonstrated that the cardiovascular safety of degludec was comparable to that of insulin glargine U100 in patients with T2D at high risk for CVD. Additionally, degludec was superior to insulin glargine U100 with respect to the risk for severe hypoglycemia. These results suggest that degludec might be preferred in patients at risk for severe hypoglycemia, including the elderly, those with CVD and/or those with chronic kidney disease.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Dailey G. Overall mortality in diabetes mellitus: where do we stand today? Diabetes Technol Ther. 2011;13(Suppl 1):S65–74.
The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53.
The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72.
Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–39.
FDA. Guidance for Industry Diabetes Mellitus — Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes. 2008. http://www.fda.gov/cder/guidance/index.htm. Accessed 3 June 2018.
Cefalu WT, Kaul S, Gerstein HC, Holman RR, Zinman B, Skyler JS, et al. Cardiovascular outcomes trials in type 2 diabetes: where do we go from here? Reflections from a diabetes care editors’ expert forum. Diabetes Care. 2017;41(1):14–31. https://doi.org/10.2337/dci17-0057.
Ratner RE, Gough SC, Mathieu C, et al. Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre-planned meta-analysis of phase 3 trials. Diabetes Obes Metab. 2013;15:175–84.
Heise T, Hermanski L, Nosek L, Feldman A, Rasmussen S, Haahr H. Insulin degludec: four times lower pharmacodynamic variability than insulin glargine under steady-state conditions in type 1 diabetes. Diabetes Obes Metab. 2012;14:859–64.
• Wysham C, Bhargava A, Chaykin L, et al. Effect of Insulin Degludec vs Insulin Glargine U100 on Hypoglycemia in Patients With Type 2 Diabetes: The SWITCH 2 Randomized Clinical Trial. JAMA. 2017;318(1):45–56. https://doi.org/10.1001/jama.2017.7117. This short-term, phase 3b study indicated lower rates of hypoglycemia with degludec vs. insulin glargine U100 which was confirmed in the much larger DEVOTE study.
Marso SP, McGuire DK, Zinman B, et al. Design of DEVOTE (trial comparing cardiovascular safety of insulin degludec vs insulin glargine in patients with type 2 diabetes at high risk of cardiovascular events) – DEVOTE 1. Am Heart J. 2016;179:175–83. https://doi.org/10.1016/j.ahj.2016.06.004.
Trial Investigators ORIGIN, Gerstein HC, Bosch J, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367(4):319–28. https://doi.org/10.1056/NEJMoa1203858.
Hilgenfeld R, Seipke G, Berchtold H, Owens DR. The evolution of insulin glargine and its continuing contribution to diabetes care. Drugs. 2014;74:911–27.
Owens DR. Insulin preparations with prolonged effect. Diab Tech and Thera. 2011;13(Suppl 1):S5–S14.
Insulin glargine. National Center for Biotechnology Information. PubChem Compound Database. https://pubchem.ncbi.nlm.nih.gov/compound/118984454#section=Pharmacology-and-Biochemistry. Accessed 21 May 2018.
Heise T, Mathieu C. Impact of the mode of protraction of basal insulin therapies on their pharmacokinetic and pharmacodynamic properties and resulting clinical outcomes. Diabetes Obes Metab. 2017;19:3–12.
Tresiba prescribing information. 2015 (http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/203314lbl.pdf).
Tresiba summary of product characteristics. 2015 (http://www.medicines.org.uk/emc/medicine/27360).
FDA Briefing Document. Insulin degludec and insulin degludec/insulin aspart treatment to improve glycemic control in patients with diabetes mellitus briefing document. 2012. [Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM327017.pdf
Insulin degludec. National Center for Biotechnology Information. PubChem Compound Database. https://pubchem.ncbi.nlm.nih.gov/compound/118984462#section=Pharmacology-and-Biochemistry. Accessed 7 May 2018.
Heise T, Norskov M, Nosek L, Kaplan K, Famulla S, Haahr HL. Insulin degludec: lower day-to-day and within-day variability in pharmacodynamic response compared with insulin glargine 300 U/mL in type 1 diabetes. Diabetes Obes Metab. 2017;19(7):1032–9.
•• Marso SP, DK MG, Zinman B, et al. Safety of degludec versus glargine in type 2 diabetes. N Engl J Med. 2017;377(20):1994–6. https://doi.org/10.1056/nejmc1712575. This robust cardiovascular outcome trial demonstrated the cardiovascular safety of degludec in high risk Type 2 diabetes patients.
Marso SP, McGuire DK, Zinman B, Poulter NR, Emerson SS, Pieber TR, et al. Efficacy and safety of degludec versus glargine in type 2 diabetes. N Engl J Med. 2017;377(8):723–32. https://doi.org/10.1056/NEJMoa1615692.
Wysham C, Bhargava A, Chaykin L, de la Rosa R, Handelsman Y, Troelsen LN, et al. Effect of insulin degludec vs insulin glargine U100 on hypoglycemia in patients with type 2 diabetes: the SWITCH 2 randomized clinical trial. JAMA. 2017;318(1):45–56. https://doi.org/10.1001/jama.2017.7117.
DeFronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–95.
American Diabetes Association. Pharmacologic approaches to glycemic treatment. Sec 8. In standards of medical Care in Diabetes – 2017. Diabetes Care. 2017;40(Suppl. 1):S64–74.
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American diabetes association and the European association for the study of diabetes. Diabetes Care. 2015;38:140–9.
Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, et al. AACE/ACE comprehensive diabetes management algorithm 2015. Endocr Pract. 2016;22:84–113.
Age-Adjusted Percentage of Adults with Diabetes Using Diabetes Medication, by Type of Medication, United States, 1997–2011. Centers for Disease Control and Prevention. https://wwwcdcgov/diabetes/statistics/meduse/fig2htm Published November 20, 2012. Accessed 13 Aug 2018.
Graveling AJ, Frier BM. Hypoglycaemia: an overview. Prim Care Diabetes. 2009;3(3):131–9. https://doi.org/10.1016/j.pcd.2009.08.007.
Fidler C, Elmelund Christensen T, Gillard S. Hypoglycemia: an overview of fear of hypoglycemia, quality-of-life, and impact on costs. J Med Econ. 2011;14(5):646–55. https://doi.org/10.3111/13696998.2011.610852.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Richard E. Pratley reports grants from Novo Nordisk, other from AstraZeneca, other from Boehringer-Ingelheim, grants from Gilead Sciences, other from GlaxoSmithKline, other from Hanmi Pharmaceutical Co., Ltd., other from Janssen Scientific Affairs, LLC, grants from Lexicon Pharmaceuticals, grants and other from Ligand Pharmaceuticals, Inc., grants and other from Lilly, grants and other from Merck, grants and other from Novo Nordisk, other from Pfizer, grants from Sanofi-Aventis US, LLC, grants and other from Takeda, other from Eisai, Inc., and other from Sanofi US Services, Inc. Except for other from Sanofi US Services, Inc., Dr. Richard Pratley’s services were paid for directly to Florida Hospital, a nonprofit organization.
Dr. Anika Bilal declares that she has no conflict of interest.
Human and Animal Rights and Informed Consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
This article is part of the Topical Collection on Macrovascular Complications in Diabetes
Rights and permissions
About this article
Cite this article
Bilal, A., Pratley, R.E. Cardiovascular Outcomes Trials Update: Insights from the DEVOTE Trial. Curr Diab Rep 18, 102 (2018). https://doi.org/10.1007/s11892-018-1086-1
Published:
DOI: https://doi.org/10.1007/s11892-018-1086-1