Abstract
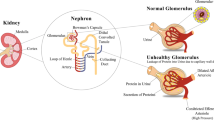
Diabetes mellitus (DM) commonly leads to progressive chronic kidney disease despite current best medical practice. The pathogenesis of diabetic kidney disease (DKD) involves a complex network of primary and secondary mechanisms with both intra-renal and systemic components. Apart from inhibition of the renin angiotensin aldosterone system, targeting individual pathogenic mediators with drug therapy has not, thus far, been proven to have high clinical value. Stem or progenitor cell therapies offer an alternative strategy for modulating complex disease processes through suppressing multiple pathogenic pathways and promoting pro-regenerative mechanisms. Mesenchymal stem cells (MSCs) have shown particular promise based on their accessibility from adult tissues and their diverse mechanisms of action including secretion of paracrine anti-inflammatory and cyto-protective factors. In this review, the progress toward clinical translation of MSC therapy for DKD is critically evaluated. Results from animal models suggest distinct potential for systemic MSC infusion to favourably modulate DKD progression. However, only a few early phase clinical trials have been initiated and efficacy in humans remains to be proven. Key knowledge gaps and research opportunities exist in this field. These include the need to gain greater understanding of in vivo mechanism of action, to identify quantifiable biomarkers of response to therapy and to define the optimal source, dose and timing of MSC administration. Given the rising prevalence of DM and DKD worldwide, continued progress toward harnessing the inherent regenerative functions of MSCs and other progenitor cells for even a subset of those affected has potential for profound societal benefits.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance, •• Of major importance
van Dieren S, Beulens JW, van der Schouw YT, Grobbee DE, Neal B. The global burden of diabetes and its complications: an emerging pandemic. Eur J Cardiovasc Prev Rehabil. 2010;17 Suppl 1:S3–8.
Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53.
World Health Organization: Diabetes Fact Sheet, 2012. Available at: http://www.whoint/mediacentre/factsheets/fs312/en/indexhtml
Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14.
de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305:2532–9.
Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164–76.
USRDS: the United States Renal Data System. Am J Kidney Dis. 2003;42(6 Suppl 5):1–230.
Chen W, Chen W, Wang H, Dong X, Liu Q, Mao H, et al. Prevalence and risk factors associated with chronic kidney disease in an adult population from southern China. Nephrol Dial Transplant. 2009;24:1205–12.
Yokoyama H, Sone H, Oishi M, Kawai K, Fukumoto Y, Kobayashi M, et al. Prevalence of albuminuria and renal insufficiency and associated clinical factors in type 2 diabetes: the Japan Diabetes Clinical Data Management study (JDDM15). Nephrol Dial Transplant. 2009;24:1212–9.
Young BA, Maynard C, Boyko EJ. Racial differences in diabetic nephropathy, cardiovascular disease, and mortality in a national population of veterans. Diabetes Care. 2003;26(8):2392–9.
Yang CW, Park JT, Kim YS, Kim YL, Lee YS, Oh YS, et al. Prevalence of diabetic nephropathy in primary care type 2 diabetic patients with hypertension: data from the Korean Epidemiology Study on Hypertension III (KEY III study). Nephrol Dial Transplant. 2011;26:3249–55.
de Boer IH, Katz R, Cao JJ, Fried LF, Kestenbaum B, Mukamal K, et al. Cystatin C, albuminuria, and mortality among older adults with diabetes. Diabetes Care. 2009;32:1833–8.
Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20:1813–21.
Emerging Risk Factors Collaboration, Seshasai SR, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–41.
Groop PH, Thomas MC, Moran JL, Waden J, Thorn LM, Makinen VP, et al. The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes. 2009;58:1651–8.
Orchard TJ, Secrest AM, Miller RG, Costacou T. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia. 2010;53:2312–9.
Afkarian M, Sachs MC, Kestenbaum B, Hirsch IB, Tuttle KR, Himmelfarb J, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24:302–8. This epidemiological study, based on the NHANES cohort from the USA, underscores the fact that the excess cardiovascular morbidity and mortality associated with type 2 DM is predominantly manifest among those with CKD.
Tancredi M, Rosengren A, Svensson AM, Kosiborod M, Pivodic A, Gudbjörnsdottir S, et al. Excess mortality among persons with type 2 diabetes. New Engl J Med. 2015;373:1720–32. This epidemiological study from Sweden demonstrated that the excess risk of all-cause and cardiovascular death in type 2 DM increased with greater severity of renal complications as well as with younger age and worse glycaemic control.
Reidy K, Kang HM, Hostetter T, Susztak K. Molecular mechanisms of diabetic kidney disease. J Clin Invest. 2014;124:2333–40. This review by leading researchers summarises recent insights into the pathophysiology of renal damage in DM.
United States Renal Data System, 2014 Annual Data Report. Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease; 2014. [cited 2015 16 June]. Available from: http://www.usrds.org/adr.aspx.
Sharma K, Karl B, Mathew AV, Gangoiti JA, Wassel CL, Saito R, et al. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J Am Soc Nephrol. 2013;24:1901–12. Using metabolomics screening of urine, the authors identified a 14-metabolite signature that was altered in DKD and that provided evidence for intra-renal mitochondrial dysfunction as an important pathophysiological abnormality.
Noh H, King GL. The role of protein kinase C activation in diabetic nephropathy. Kidney Int Suppl. 2007;106:S49–53.
Derubertis FR, Craven PA. Activation of protein kinase C in glomerular cells in diabetes. Mechanisms and potential links to the pathogenesis of diabetic glomerulopathy. Diabetes. 1994;43:1–8.
Hayashi T, Takai S, Yamashita C. Impact of the renin-angiotensin-aldosterone-system on cardiovascular and renal complications in diabetes mellitus. Curr Vasc Pharmacol. 2010;8:189–97.
Vargas SL, Toma I, Kang JJ, Meer EJ, Peti-Peterdi J. Activation of the succinate receptor GPR91 in macula densa cells causes renin release. J Am Soc Nephrol. 2009;20:1002–11.
Wolf G, Mueller E, Stahl RA, Ziyadeh FN. Angiotensin II-induced hypertrophy of cultured murine proximal tubular cells is mediated by endogenous transforming growth factor-beta. J Clin Invest. 1993;92:1366–72.
Kalinyak JE, Sechi LA, Griffin CA, Don BR, Tavangar K, Kraemer FB, et al. The renin-angiotensin system in streptozotocin-induced diabetes mellitus in the rat. J Am Soc Nephrol. 1993;4:1337–45.
Tesch GH. MCP-1/CCL2: a new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2008;294:F697–701.
Wada T, Yokoyama H, Matsushima K, Kobayashi K. Monocyte chemoattractant protein-1: does it play a role in diabetic nephropathy? Nephrol Dial Transplant. 2003;18:457–9.
Yadav A, Vallabu S, Arora S, Tandon P, Slahan D, Teichberg S, et al. ANG II promotes autophagy in podocytes. Am J Physiol Cell Physiol. 2010;299:C488–96.
Sun L, Kanwar YS. Relevance of TNF-alpha in the context of other inflammatory cytokines in the progression of diabetic nephropathy. Kidney Int. 2015;88:662–5. This recent review focusses on clinical and experimental evidence for the role of TNF-α in the pathogenesis of DKD.
Rodriguez-Iturbe B, Pons H, Herrera-Acosta J, Johnson RJ. Role of immunocompetent cells in nonimmune renal diseases. Kidney Int. 2001;59:1626–40.
Di Paolo S, Gesualdo L, Ranieri E, Grandaliano G, Schena FP. High glucose concentration induces the overexpression of transforming growth factor-beta through the activation of a platelet-derived growth factor loop in human mesangial cells. Am J Pathol. 1996;149:2095–106.
Schena FP, Gesualdo L. Pathogenetic mechanisms of diabetic nephropathy. J Am Soc Nephrol. 2005;16 Suppl 1:S30–3.
Furuta T, Saito T, Ootaka T, Soma J, Obara K, Abe K, et al. The role of macrophages in diabetic glomerulosclerosis. Am J Kidney Dis. 1993;21:480–5.
Nguyen D, Ping F, Mu W, Hill P, Atkins RC, Chadban SJ. Macrophage accumulation in human progressive diabetic nephropathy. Nephrol (Carlton). 2006;11:226–31.
Lim A. Diabetic nephropathy—complications and treatment. Int J Nephrol Renovasc Dis. 2014;7:361–81.
Bending JJ, Lobo-Yeo A, Vergani D, Viberti GC. Proteinuria and activated T-lymphocytes in diabetic nephropathy. Diabetes. 1988;37:507–11.
Ilan Y, Maron R, Tukpah AM, Maioli TU, Murugaiyan G, Yang K, et al. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc Natl Acad Sci U S A. 2010;107:9765–70.
Eller K, Kirsch A, Wolf AM, Sopper S, Tagwerker A, Stanzl U, et al. Potential role of regulatory T cells in reversing obesity-linked insulin resistance and diabetic nephropathy. Diabetes. 2011;60:2954–62.
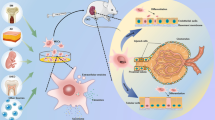
Griffin MD, Elliman SJ, Cahill E, English K, Ceredig R, Ritter T. Concise review: adult mesenchymal stromal cell therapy for inflammatory diseases: how well are we joining the dots? Stem Cells. 2013;31:2033–41. This recent review provides a critical evaluation of current progress in understanding the anti-inflammatory properties of MSCs and in developing successful MSC therapies for human inflammatory diseases with emphasis on key challenges in the translational process.
Alpers CE, Hudkins KL. Mouse models of diabetic nephropathy. Curr Opin Nephrol Hypertens. 2011;20:278–84.
Pan XH, Yang XY, Yao X, Sun XM, Zhu L, Wang JX, et al. Bone-marrow mesenchymal stem cell transplantation to treat diabetic nephropathy in tree shrews. Cell Biochem Funct. 2014;32:453–63.
Fan Y, Huang ZY, Cao CC, Chen CS, Chen YX, Fan DD, et al. Genome of the Chinese tree shrew. Nat Commun. 2013;4:1426.
Xu L, Chen SY, Nie WH, Jiang XL, Yao YG. Evaluating the phylogenetic position of Chinese tree shrew (Tupaia belangeri chinensis) based on complete mitochondrial genome: implication for using tree shrew as an alternative experimental animal to primates in biomedical research. J Genet Genomics. 2012;39:131–7.
Rabb GB, Getty RE, Williamson WM, Lombard LS. Spontaneous diabetes mellitus in tree shrews, Urogale everetti. Diabetes. 1966;15:327–30.
Wu X, Chang Q, Zhang Y, Zou X, Chen L, Zhang L, et al. Relationships between body weight, fasting blood glucose concentration, sex and age in tree shrews (Tupaia belangeri chinensis). J Anim Physiol Anim Nutr (Berl). 2013;97:1179–88.
Gurley SB, Clare SE, Snow KP, Hu A, Meyer TW, Coffman TM. Impact of genetic background on nephropathy in diabetic mice. Am J Physiol Renal Physiol. 2006;290:F214–22.
Kong LL, Wu H, Cui WP, Zhou WH, Luo P, Sun J, et al. Advances in murine models of diabetic nephropathy. J Diabetes Res. 2013;2013:797548.
Zhang L, Li K, Liu X, Li D, Luo C, Fu B, et al. Repeated systemic administration of human adipose-derived stem cells attenuates overt diabetic nephropathy in rats. Stem Cell Dev. 2013;22:3074–86. In this pre-clinical study, repeated intravenous administration of human adipose-derived MSCs resulted in improvements to albuminuria and structural features of renal damage in an accelerated model of DKD in rat.
Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci U S A. 2006;103:17438–43.
Devarapu SK, Junhui X, Darisipudi M, Rocanin Arjo A, Anders HJ. CD362+ mesenchymal stem cell treatment of kidney disease in type 2 diabetic Lepr db/db mice. Nephrol Dial Transplant. 2015;30 suppl 3:iii223–4.
Tay YC, Wang Y, Kairaitis L, Rangan GK, Zhang C, Harris DC. Can murine diabetic nephropathy be separated from superimposed acute renal failure? Kidney Int. 2005;68:391–8.
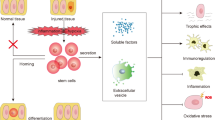
Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053–67.
Herrera MB, Bussolati B, Bruno S, Fonsato V, Romanazzi GM, Camussi G. Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int J Mol Med. 2004;14:1035–41.
Ezquer F, Ezquer M, Simon V, Pardo F, Yanez A, Carpio D, et al. Endovenous administration of bone-marrow-derived multipotent mesenchymal stromal cells prevents renal failure in diabetic mice. Biol Blood Marrow Transplant. 2009;15:1354–65.
Kraynak AR, Storer RD, Jensen RD, Kloss MW, Soper KA, Clair JH, et al. Extent and persistence of streptozotocin-induced DNA damage and cell proliferation in rat kidney as determined by in vivo alkaline elution and BrdUrd labeling assays. Toxicol Appl Pharmacol. 1995;135:279–86.
Tesch GH, Allen TJ. Rodent models of streptozotocin-induced diabetic nephropathy. Nephrol (Carlton). 2007;12:261–6.
Hudkins KL, Pichaiwong W, Wietecha T, Kowalewska J, Banas MC, Spencer MW, et al. BTBR Ob/Ob mutant mice model progressive diabetic nephropathy. J Am Soc Nephrol. 2010;21:1533–42.
Xu J, Huang Y, Li F, Zheng S, Epstein PN. FVB mouse genotype confers susceptibility to OVE26 diabetic albuminuria. Am J Physiol Renal Physiol. 2010;299:F487–94.
Zhao HJ, Wang S, Cheng H, Zhang MZ, Takahashi T, Fogo AB, et al. Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. J Am Soc Nephrol. 2006;17:2664–9.
Fang Y, Tian X, Bai S, Fan J, Hou W, Tong H, et al. Autologous transplantation of adipose-derived mesenchymal stem cells ameliorates streptozotocin-induced diabetic nephropathy in rats by inhibiting oxidative stress, pro-inflammatory cytokines and the p38 MAPK signaling pathway. Int J Mol Med. 2012;30:85–92.
Ezquer FE, Ezquer ME, Parrau DB, Carpio D, Yanez AJ, Conget PA. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant. 2008;14:631–40.
Abdel Aziz MT, Wassef MA, Ahmed HH, Rashed L, Mahfouz S, Aly MI, et al. The role of bone marrow derived-mesenchymal stem cells in attenuation of kidney function in rats with diabetic nephropathy. Diabetol Metab Syndr. 2014;6:34.
Lv S, Cheng J, Sun A, Li J, Wang W, Guan G, et al. Mesenchymal stem cells transplantation ameliorates glomerular injury in streptozotocin-induced diabetic nephropathy in rats via inhibiting oxidative stress. Diabetes Res Clin Pract. 2014;104:143–54.
Lv SS, Liu G, Wang JP, Wang WW, Cheng J, Sun AL, et al. Mesenchymal stem cells transplantation ameliorates glomerular injury in streptozotocin-induced diabetic nephropathy in rats via inhibiting macrophage infiltration. Int Immunopharmacol. 2013;17:275–82. In this study, administration of autologous bone marrow-derived MSCs to rats with streptozotocin-induced DM resulted in improvements in the severity of DKD with reduced intra-renal expression of MCP-1 and pro-inflammatory cytokines as well as reduced renal macrophage infiltration.
Wang S, Li Y, Zhao J, Zhang J, Huang Y. Mesenchymal stem cells ameliorate podocyte injury and proteinuria in a type 1 diabetic nephropathy rat model. Biol Blood Marrow Transplant. 2013;19:538–46.
Zhou H, Tian HM, Long Y, Zhang XX, Zhong L, Deng L, et al. Mesenchymal stem cells transplantation mildly ameliorates experimental diabetic nephropathy in rats. Chin Med J. 2009;122:2573–9.
Li D, Wang N, Zhang L, Hanyu Z, Xueyuan B, Fu B, et al. Mesenchymal stem cells protect podocytes from apoptosis induced by high glucose via secretion of epithelial growth factor. Stem Cell Res Ther. 2013;4:103. This mechanistic study provided evidence that epithelial growth factor secreted by human adipose-derived MSCs protect podocytes from hyperglycaemia-induced apoptosis.
Park JH, Hwang I, Hwang SH, Han H, Ha H. Human umbilical cord blood-derived mesenchymal stem cells prevent diabetic renal injury through paracrine action. Diabetes Res Clin Pract. 2012;98:465–73.
Park JH, Park J, Hwang SH, Han H, Ha H. Delayed treatment with human umbilical cord blood-derived stem cells attenuates diabetic renal injury. Transplant Proc. 2012;44:1123–6.
Davey GC, Patil SB, O'Loughlin A, O'Brien T. Mesenchymal stem cell-based treatment for microvascular and secondary complications of diabetes mellitus. Front Endocrinol. 2014;5:86.
Zhang Y, Ye C, Wang G, Gao Y, Tan K, Zhuo Z, et al. Kidney-targeted transplantation of mesenchymal stem cells by ultrasound-targeted microbubble destruction promotes kidney repair in diabetic nephropathy rats. BioMed Res Int. 2013;2013:526367.
Ezquer F, Ezquer M, Contador D, Ricca M, Simon V, Conget P. The anti-diabetic effect of mesenchymal stem cells is unrelated to their transdifferentiation potential but to their capability to restore Th1/Th2 balance and to modify the pancreatic microenvironment. Stem Cells. 2012;30:1664–74.
Hess D, Li L, Martin M, Sakano S, Hill D, Strutt B, et al. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat Biotechnol. 2003;21:763–70.
Madec AM, Mallone R, Afonso G, Abou Mrad E, Mesnier A, Eljaafari A, et al. Mesenchymal stem cells protect NOD mice from diabetes by inducing regulatory T cells. Diabetologia. 2009;52:1391–9.
Ezquer ME, Ezquer FE, Arango-Rodriguez ML, Conget PA. MSC transplantation: a promising therapeutic strategy to manage the onset and progression of diabetic nephropathy. Biol Res. 2012;45:289–96.
Marquez-Curtis LA, Janowska-Wieczorek A. Enhancing the migration ability of mesenchymal stromal cells by targeting the SDF-1/CXCR4 axis. Biomed Res Int. 2013;2013:561098.
Wu S, Li L, Wang G, Shen W, Xu Y, Liu Z, et al. Ultrasound-targeted stromal cell-derived factor-1-loaded microbubble destruction promotes mesenchymal stem cell homing to kidneys in diabetic nephropathy rats. Int J Nanomedicine. 2014;9:5639–51. The authors of this innovative study demonstrated that release of SDF-1 in the kidney by USTMD resulted in increased homing of intravenously administered MSCs and improved MSC efficacy in a rat model of DKD.
Chen S, Ding JH, Bekeredjian R, Yang BZ, Shohet RV, Johnston SA, et al. Efficient gene delivery to pancreatic islets with ultrasonic microbubble destruction technology. Proc Natl Acad Sci U S A. 2006;103:8469–74.
Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet. 1982;1:1430–2.
Parving HH, Oxenboll B, Svendsen PA, Christiansen JS, Andersen AR. Early detection of patients at risk of developing diabetic nephropathy. A longitudinal study of urinary albumin excretion. Acta Endocrinol (Copenh). 1982;100:550–5.
Mogensen CE, Christensen CK. Predicting diabetic nephropathy in insulin-dependent patients. N Engl J Med. 1984;311:89–93.
Garrahy A, Tormey WP. Pitfalls of the urinary albumin creatinine ratio in detection of early diabetic kidney disease. Ir Med J. 2015;108:102–3.
Ismail N, Becker B, Strzelczyk P, Ritz E. Renal disease and hypertension in non-insulin-dependent diabetes mellitus. Kidney Int. 1999;55:1–28.
Caramori ML, Fioretto P, Mauer M. The need for early predictors of diabetic nephropathy risk: is albumin excretion rate sufficient? Diabetes. 2000;49:1399–408.
Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–30.
Currie G, McKay G, Delles C. Biomarkers in diabetic nephropathy: present and future. World J Diabetes. 2014;5:763–76.
Fassett RG, Venuthurupalli SK, Gobe GC, Coombes JS, Cooper MA, Hoy WE. Biomarkers in chronic kidney disease: a review. Kidney Int. 2011;80:806–21.
Pena MJ, Lambers Heerspink HJ, Hellemons ME, Friedrich T, Dallmann G, Lajer M, et al. Urine and plasma metabolites predict the development of diabetic nephropathy in individuals with type 2 diabetes mellitus. Diabet Med. 2014;31(9):1138–47.
Navarro JF, Mora C, Macıéa M, Garcıéa J. Inflammatory parameters are independently associated with urinary albumin in type 2 diabetes mellitus. Am J Kidney Dis. 2003;42:53–61.
Navarro JF, Mora C, Gomez M, Muros M, Lopez-Aguilar C, Garcia J. Influence of renal involvement on peripheral blood mononuclear cell expression behaviour of tumour necrosis factor- and interleukin-6 in type 2 diabetic patients. Nephrol Dial Transplant. 2007;23:919–26.
Navarro JF, Mora C, Muros M, Garcia J. Urinary tumour necrosis factor- excretion independently correlates with clinical markers of glomerular and tubulointerstitial injury in type 2 diabetic patients. Nephrol Dial Transplant. 2006;21:3428–34.
Niewczas MA, Ficociello LH, Johnson AC, Walker W, Rosolowsky ET, Roshan B, et al. Serum concentrations of markers of TNF and Fas-mediated pathways and renal function in nonproteinuric patients with type 1 diabetes. Clin J Am Soc Nephrol. 2009;4:62–70.
Gohda T, Niewczas MA, Ficociello LH, Walker WH, Skupien J, Rosetti F, et al. Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol. 2012;23:516–24.
Niewczas MA, Gohda T, Skupien J, Smiles AM, Walker WH, Rosetti F, et al. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol. 2012;23:507–15. This biomarker study provides strong evidence that elevated serum sTNFR 1 and 2 concentrations in subjects with type 2 diabetes mellitus predict progression to ESRD.
Christou GA, Kiortsis DN. The role of adiponectin in renal physiology and development of albuminuria. J Endocrinol. 2014;221:R49–61.
Sharma K, Ramachandrarao S, Qiu G, Usui HK, Zhu Y, Dunn SR, et al. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest. 2008;118:1645–56.
Ohashi N, Kato A, Misaki T, Sakakima M, Fujigaki Y, Yamamoto T, et al. Association of serum adiponectin levels with all-cause mortality in hemodialysis patients. Intern Med. 2008;47:485–91.
Nakamaki S, Satoh H, Kudoh A, Hayashi Y, Hirai H, Watanabe T. Adiponectin reduces proteinuria in streptozotocin-induced diabetic Wistar rats. Exp Biol Med. 2011;236:614–20.
Tsioufis C, Dimitriadis K, Chatzis D, Vasiliadou C, Tousoulis D, Papademetriou V, et al. Relation of microalbuminuria to adiponectin and augmented C-reactive protein levels in men with essential hypertension. Am J Cardiol. 2005;96:946–51.
Barlovic DP, Zaletel J, Prezelj J. Association between adiponectin and low-grade albuminuria is BMI-dependent in type 2 diabetes. Kidney Blood Press Res. 2010;33:405–10.
Barlovic DP, Zaletel J, Prezelj J. Adipocytokines are associated with renal function in patients with normal range glomerular filtration rate and type 2 diabetes. Cytokine. 2009;46:142–5.
Tamba S, Nakatsuji H, Kishida K, Noguchi M, Ogawa T, Okauchi Y, et al. Relationship between visceral fat accumulation and urinary albumin-creatinine ratio in middle-aged Japanese men. Atherosclerosis. 2010;211:601–5.
Jorsal A, Petersen EH, Tarnow L, Hess G, Zdunek D, Frystyk J, et al. Urinary adiponectin excretion rises with increasing albuminuria in type 1 diabetes. J Diabetes Complications. 2013;27:604–8.
Fujita H, Morii T, Koshimura J, Ishikawa M, Kato M, Miura T, et al. Possible relationship between adiponectin and renal tubular injury in diabetic nephropathy. Endocr J. 2006;53:745–52.
Koshimura J, Fujita H, Narita T, Shimotomai T, Hosoba M, Yoshioka N, et al. Urinary adiponectin excretion is increased in patients with overt diabetic nephropathy. Biochem Biophys Res Commun. 2004;316:165–9.
Looker HC, Krakoff J, Funahashi T, Matsuzawa Y, Tanaka S, Nelson RG, et al. Adiponectin concentrations are influenced by renal function and diabetes duration in Pima Indians with type 2 diabetes. J Clin Endocrinol Metab. 2004;89:4010–7.
Costacou T, Zgibor JC, Evans RW, Otvos J, Lopes-Virella MF, Tracy RP, et al. The prospective association between adiponectin and coronary artery disease among individuals with type 1 diabetes. The Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia. 2005;48:41–8.
Frystyk J, Tarnow L, Hansen TK, Parving HH, Flyvbjerg A. Increased serum adiponectin levels in type 1 diabetic patients with microvascular complications. Diabetologia. 2005;48:1911–8.
Schalkwijk CG, Chaturvedi N, Schram MT, Fuller JH, Stehouwer CD, Group EPCS. Adiponectin is inversely associated with renal function in type 1 diabetic patients. J Clin Endocrinol Metab. 2006;91:129–35.
Kacso IM, Bondor CI, Kacso G. Plasma adiponectin is related to the progression of kidney disease in type 2 diabetes patients. Scand J Clin Lab Invest. 2012;72:333–9.
Saraheimo M, Forsblom C, Thorn L, Waden J, Rosengard-Barlund M, Heikkila O, et al. Serum adiponectin and progression of diabetic nephropathy in patients with type 1 diabetes. Diabetes Care. 2008;31:1165–9.
Panduru NM, Saraheimo M, Forsblom C, Thorn LM, Gordin D, Waden J, et al. Urinary adiponectin is an independent predictor of progression to end-stage renal disease in patients with type 1 diabetes and diabetic nephropathy. Diabetes Care. 2015;38:883–90. This recent study from the FinnDiane study group demonstrated that urinary adiponectin independently predicts progression from macro-albuminuria to ESRD in type 1 DM.
Fu W-J, Li B-L, Wang S-B, Chen M-L, Deng R-T, Ye C-Q, et al. Changes of the tubular markers in type 2 diabetes mellitus with glomerular hyperfiltration. Diabetes Res Clin Pract. 2012;95:105–9.
Yang Y-H, He X-J, Chen S-R, Wang L, Li E-M, Xu L-Y. Changes of serum and urine neutrophil gelatinase-associated lipocalin in type-2 diabetic patients with nephropathy: one year observational follow-up study. Endocrine. 2009;36:45–51.
Nielsen SE, Sugaya T, Hovind P, Baba T, Parving HH, Rossing P. Urinary liver-type fatty acid-binding protein predicts progression to nephropathy in type 1 diabetic patients. Diabetes Care. 2010;33:1320–4.
Bolignano D, Lacquaniti A, Coppolino G, Donato V, Fazio MR, Nicocia G, et al. Neutrophil gelatinase-associated lipocalin as an early biomarker of nephropathy in diabetic patients. Kidney Blood Press Res. 2009;32:91–8.
Nielsen SE, Reinhard H, Zdunek D, Hess G, Gutiérrez OM, Wolf M, et al. Tubular markers are associated with decline in kidney function in proteinuric type 2 diabetic patients. Diabetes Res Clin Pract. 2012;97:71–6.
Chou K-M, Lee C-C, Chen C-H, Sun C-Y. Clinical value of NGAL, L-FABP and albuminuria in predicting GFR decline in type 2 diabetes mellitus patients. PLoS One. 2013;8:e54863.
Conway BR, Manoharan D, Manoharan D, Jenks S, Dear JW, McLachlan S, et al. Measuring urinary tubular biomarkers in type 2 diabetes does not add prognostic value beyond established risk factors. Kidney Int. 2012;82:812–8.
Lee CH, Lam KS. Biomarkers of progression in diabetic nephropathy: the past, present and future. J Diabetes Investig. 2015;6:247–9.
Titan SM, Zatz R, Graciolli FG, dos Reis LM, Barros RT, Jorgetti V, et al. FGF-23 as a predictor of renal outcome in diabetic nephropathy. Clin J Am Soc Nephrol. 2011;6:241–7.
Lee CH, Hui EY, Woo YC, Yeung CY, Chow WS, Yuen MM, et al. Circulating fibroblast growth factor 21 levels predict progressive kidney disease in subjects with type 2 diabetes and normoalbuminuria. J Clin Endocrinol Metab. 2015;100:1368–75.
Sabbisetti VS, Waikar SS, Antoine DJ, Smiles A, Wang C, Ravisankar A, et al. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J Am Soc Nephrol. 2014;25:2177–86.
van Timmeren MM, van den Heuvel MC, Bailly V, Bakker SJL, van Goor H, Stegeman CA. Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol. 2007;212:209–17.
Vaidya VS, Niewczas MA, Ficociello LH, Johnson AC, Collings FB, Warram JH, et al. Regression of microalbuminuria in type 1 diabetes is associated with lower levels of urinary tubular injury biomarkers, kidney injury molecule-1, and N-acetyl-beta-D-glucosaminidase. Kidney Int. 2011;79:464–70.
Nielsen SE, Andersen S, Zdunek D, Hess G, Parving HH, Rossing P. Tubular markers do not predict the decline in glomerular filtration rate in type 1 diabetic patients with overt nephropathy. Kidney Int. 2011;79:1113–8.
Nielsen SE, Rossing K, Hess G, Zdunek D, Jensen BR, Parving H-H, et al. The effect of RAAS blockade on markers of renal tubular damage in diabetic nephropathy: u-NGAL, u-KIM1 and u-LFABP. Scand J Clin Lab Invest. 2012;72:137–42.
Looker HC, Colombo M, Hess S, Brosnan MJ, Farran B, Dalton RN, et al. Biomarkers of rapid chronic kidney disease progression in type 2 diabetes. Kidney Int. 2015;88(4):888–96. In this study the authors screened a large number of putative biomarkers for associations with DKD progression. A panel of 14 biomarkers including FGF-21, symmetric to asymmetric dimethylarginine ratio, β2-microglobulin, C16-acylcarnitine and KIM-1 was identified as improving the prediction of rapidly progressive decline in eGFR when added to clinical predictors.
Hovind P, Tarnow L, Oestergaard PB, Parving H-H. Elevated vascular endothelial growth factor in type 1 diabetic patients with diabetic nephropathy. Kidney Int. 2000;57(s75):56–61.
Kim NH, Kim KB, Kim DL, Kim SG, Choi KM, Baik SH, et al. Plasma and urinary vascular endothelial growth factor and diabetic nephropathy in type 2 diabetes mellitus. Diabet Med. 2004;21:545–51.
Kim NH, Oh JH, Seo JA, Lee KW, Kim SG, Choi KM, et al. Vascular endothelial growth factor (VEGF) and soluble VEGF receptor FLT-1 in diabetic nephropathy. Kidney Int. 2005;67:167–77.
Santilli F, Spagnoli A, Mohn A, Tumini S, Verrotti A, Cipollone F, et al. Increased vascular endothelial growth factor serum concentrations may help to identify patients with onset of type 1 diabetes during childhood at risk for developing persistent microalbuminuria. J Clin Endocrinol Metab. 2001;86:3871–6.
D'Angio CT, Ambati J, Phelps DL. Do urinary levels of vascular endothelial growth factor predict proliferative retinopathy? Curr Eye Res. 2001;22:90–4.
Fiseha T. Urinary biomarkers for early diabetic nephropathy in type 2 diabetic patients. Biomark Res. 2015;3:16.
Robles-Osorio ML, Sabath E. Tubular dysfunction and non-albuminuric renal disease in subjects with type 2 diabetes mellitus. Rev Invest Clin. 2014;66:234–9.
Hong CY, Hughes K, Chia KS, Ng V, Ling SL. Urinary 1-microglobulin as a marker of nephropathy in type 2 diabetic Asian subjects in Singapore. Diabetes Care. 2003;26:338–42.
Petrica L, Vlad A, Gluhovschi G, Gadalean F, Dumitrascu V, Gluhovschi C, et al. Proximal tubule dysfunction is associated with podocyte damage biomarkers nephrin and vascular endothelial growth factor in type 2 diabetes mellitus patients: a cross-sectional study. PLoS One. 2014;9:e112538.
Petrica L, Petrica M, Vlad A, Jianu DC, Gluhovschi G, Ianculescu C, et al. Proximal tubule dysfunction is dissociated from endothelial dysfunction in normoalbuminuric patients with type 2 diabetes mellitus: a cross-sectional study. Nephron Clin Pract. 2011;118:c155–64.
Fliser D, Novak J, Thongboonkerd V, Argiles A, Jankowski V, Girolami MA, et al. Advances in urinary proteome analysis and biomarker discovery. J Am Soc Nephrol. 2007;18:1057–71.
Mischak H, Coon JJ, Novak J, Weissinger EM, Schanstra JP, Dominiczak AF. Capillary electrophoresis-mass spectrometry as a powerful tool in biomarker discovery and clinical diagnosis: an update of recent developments. Mass Spectrom Rev. 2009;28:703–24.
Mischak H, Delles C, Klein J, Schanstra JP. Urinary proteomics based on capillary electrophoresis-coupled mass spectrometry in kidney disease: discovery and validation of biomarkers, and clinical application. Adv Chronic Kidney Dis. 2010;17:493–506.
Good DM, Zurbig P, Argiles A, Bauer HW, Behrens G, Coon JJ, et al. Naturally occurring human urinary peptides for use in diagnosis of chronic kidney disease. Mol Cell Proteomics. 2010;9:2424–37.
Argiles A, Siwy J, Duranton F, Gayrard N, Dakna M, Lundin U, et al. CKD273, a new proteomics classifier assessing CKD and its prognosis. PLoS One. 2013;8:e62837.
Siwy J, Schanstra JP, Argiles A, Bakker SJL, Beige J, Boucek P, et al. Multicentre prospective validation of a urinary peptidome-based classifier for the diagnosis of type 2 diabetic nephropathy. Nephrol Dial Transplant. 2014;29:1563–70. This study provided the first validation of the CKD273 urinary proteome-based classifier of DKD in a multicentre prospective setting involving type 2 diabetic subjects.
Roscioni SS, de Zeeuw D, Hellemons ME, Mischak H, Zürbig P, Bakker SJL, et al. A urinary peptide biomarker set predicts worsening of albuminuria in type 2 diabetes mellitus. Diabetologia. 2012;56:259–67.
Zurbig P, Jerums G, Hovind P, MacIsaac RJ, Mischak H, Nielsen SE, et al. Urinary proteomics for early diagnosis in diabetic nephropathy. Diabetes. 2012;61:3304–13.
Cantaluppi V, Biancone L, Quercia A, Deregibus MC, Segoloni G, Camussi G. Rationale of mesenchymal stem cell therapy in kidney injury. Am J Kidney Dis. 2013;61:300–9.
Packham DK, Fraser I, Kerr PG, Lichliter J, Itescu S, Skerrett D, et al. Mesenchymal stem cell therapy for diabetic nephropathy: a phase 2 randomized controlled trial. Diabetes. 2015;64(Suppl 1A):LB6. Although only reported in abstract form to date, the preliminary results for this completed Phase I/II trial provide key insights into the safety and potential efficacy of MSC therapy for established DKD.
Acknowledgments
The authors are supported by grants from the European Commission [Horizon 2020 Collaborative Health Project NEPHSTROM (grant number 634086; TPG, WPM, NI, TO’B, MDG) and FP7 Collaborative Health Project VISICORT (grant number 602470; MDG)] and from Science Foundation Ireland [REMEDI Strategic Research Cluster (grant number 09/SRC-B1794; TO’B, MDG) and CÚRAM Research Centre (grant number 13/RC/2073; TO’B, MDG)] and by the European Regional Development Fund. TPG is supported by a Hardiman Scholarship from the College of Medicine, Nursing and Health Science of the National University of Ireland, Galway.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Tomás P. Griffin declares that he has no conflict of interest.
William Patrick Martin reports grant support from the European Commission.
Nahidul Islam reports grant support from the European Commission.
Timothy O’Brien reports grants from the European Commission, Science Foundation Ireland, and Medtronic. He reports other from the European Regional Development Fund, Orbsen Therapeutics and Onkimmune. He reports personal fees from Merck Sharp and Dohme, Sanofi Regeneron, Eli Lilly and Novo Nordisk.
Matthew D. Griffin reports grants from the European Commission, Science Foundation Ireland and Randox Teoranta; and other from the European Regional Development Fund,
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Rights and permissions
About this article
Cite this article
Griffin, T.P., Martin, W.P., Islam, N. et al. The Promise of Mesenchymal Stem Cell Therapy for Diabetic Kidney Disease. Curr Diab Rep 16, 42 (2016). https://doi.org/10.1007/s11892-016-0734-6
Published:
DOI: https://doi.org/10.1007/s11892-016-0734-6