Abstract
Purpose of Review
To summarize the current knowledge on the role that B lymphocytes play in heart failure.
Recent Findings
Several studies from murine models have shown that B cells modulate cardiac adaptation to injury and ultimately affect the degree of cardiac dysfunction after acute ischemic damage. In addition, a B cell–modulating small molecule was recently shown to have beneficial effects in humans with heart failure with preserved ejection fraction.
Summary
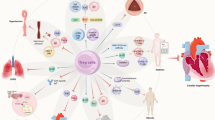
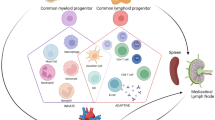
B lymphocytes are specialized immune cells present in all jawed vertebrates. They are characteristically known for their ability to produce antibodies, but they have other functions and are important players in virtually all forms of immune responses. A growing body of evidence indicates that B cells are intimately connected with the heart and that B cell dysregulation might play a role in the pathogenesis and progression of both heart failure with reduced ejection fraction and heart failure with preserved ejection fraction. B cells are therefore gathering attention as potential targets for the development of novel immunomodulatory-based treatments for heart failure.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Parra D, Takizawa F, Sunyer JO. Evolution of B cell immunity. Annu Rev Anim Biosci. 2013;1:65–97.
LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112(5):1570–80.
Chen X, Jensen PE. The role of B lymphocytes as antigen-presenting cells. Arch Immunol Ther Exp (Warsz). 2008;56(2):77–83.
Fillatreau S. Cytokine-producing B cells as regulators of pathogenic and protective immune responses. Ann Rheum Dis. 2013;72(suppl 2):ii80.
Kantor AB, Herzenberg LA. Origin of murine B cell lineages. Annu Rev Immunol. 1993;11:501–38.
Naradikian MS, et al. Understanding B cell biology. In: Bosch X, Ramos-Casals M, Khamashta MA, editors., et al., Drugs targeting B-cells in autoimmune diseases. Basel: Springer Basel; 2014. p. 11–35.
Kawahara T, et al. Peritoneal cavity B cells are precursors of splenic IgM natural antibody-producing cells. J Immunol. 2003;171(10):5406–14.
Yenson V, Baumgarth N. Purification and immune phenotyping of B-1 cells from body cavities of mice. Methods Mol Biol. 2014;1190:17–34.
Montecino-Rodriguez E, Dorshkind K. B-1 B cell development in the fetus and adult. Immunity. 2012;36(1):13–21.
•• Adamo L, et al. Modulation of subsets of cardiac B lymphocytes improves cardiac function after acute injury. JCI Insight. 2018;3(11). Findings from this study demonstrate for the first time that small molecule–mediate modulation of B cells improves cardiac function after acute ischemic injury and therefore point at B cells as a viable therapeutic target for the prevention of heart failure after myocardial infarction.
Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol. 2013;13(2):118–32.
Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381(6585):751–8.
Bos NA, et al. Serum immunoglobulin levels and naturally occurring antibodies against carbohydrate antigens in germ-free BALB/c mice fed chemically defined ultrafiltered diet. Eur J Immunol. 1989;19(12):2335–9.
Palma J, et al. Natural antibodies - facts known and unknown. Cent Eur J Immunol. 2018;43(4):466–75.
Heesters BA, et al. Antigen presentation to B cells. Trends Immunol. 2016;37(12):844–54.
Diaz M, Casali P. Somatic immunoglobulin hypermutation. Curr Opin Immunol. 2002;14(2):235–40.
Reth M. Antigen receptors on B lymphocytes. Annu Rev Immunol. 1992;10:97–121.
Kurosaki T, Kometani K, Ise W. Memory B cells. Nat Rev Immunol. 2015;15(3):149–59.
Roche PA, Furuta K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat Rev Immunol. 2015;15(4):203–16.
Katkere B, Rosa S, Drake JR. The Syk-binding ubiquitin ligase c-Cbl mediates signaling-dependent B cell receptor ubiquitination and B cell receptor-mediated antigen processing and presentation. J Biol Chem. 2012;287(20):16636–44.
Adler LN, et al. The other function: class II-restricted antigen presentation by B cells. Front Immunol. 2017;8:319–319.
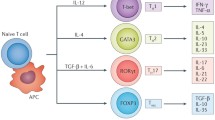
Duddy ME, Alter A, Bar-Or A. Distinct profiles of human B cell effector cytokines: a role in immune regulation? J Immunol. 2004;172(6):3422–7.
Shen P, Fillatreau S. Antibody-independent functions of B cells: a focus on cytokines. Nat Rev Immunol. 2015;15(7):441–51.
•• Zouggari Y, et al. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat Med. 2013;19(10):1273–80. Findings from this study demonstrate for the first time a functional connection between B lymphocytes and the heart.
Chousterman BG, Swirski FK. Innate response activator B cells: origins and functions. Int Immunol. 2015;27(10):537–41.
Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42(4):607–12.
Chimen M, et al. Homeostatic regulation of T cell trafficking by a B cell-derived peptide is impaired in autoimmune and chronic inflammatory disease. Nat Med. 2015;21(5):467–75.
Magee CN, Boenisch O, Najafian N. The role of costimulatory molecules in directing the functional differentiation of alloreactive T helper cells. Am J Transplant. 2012;12(10):2588–600.
Gowans JL, Knight EJ. The route of re-circulation of lymphocytes in the rat. Proc R Soc Lond B Biol Sci. 1964;159:257–82.
Kunkel EJ, Butcher EC. Chemokines and the tissue-specific migration of lymphocytes. Immunity. 2002;16(1):1–4.
Tanaka T, et al. Molecular determinants controlling homeostatic recirculation and tissue-specific trafficking of lymphocytes. Int Arch Allergy Immunol. 2004;134(2):120–34.
Adamo L, et al. Modulation of subsets of cardiac B lymphocytes improves cardiac function after acute injury. JCI Insight. 2018;3(11).
Bonner F, et al. Resident cardiac immune cells and expression of the ectonucleotidase enzymes CD39 and CD73 after ischemic injury. PLoS One. 7(4):e34730.
Rocha-Resende C, et al. Developmental changes in myocardial B cells mirror changes in B cells associated with different organs. JCI Insight, 2020;5(16).
Horckmans M, et al. Pericardial adipose tissue regulates granulopoiesis, fibrosis and cardiac function after myocardial infarction. Circulation. 2017.
Wu L, et al. IL-10-producing B cells are enriched in murine pericardial adipose tissues and ameliorate the outcome of acute myocardial infarction. Proc Natl Acad Sci U S A. 2019;116(43):21673–84.
Adamo L, et al. Myocardial B cells are a subset of circulating lymphocytes with delayed transit through the heart. JCI Insight. 2020;5(3).
• Rocha-Resende C, Pani F, Adamo L. B cells modulate the expression of MHC-II on cardiac CCR2(-) macrophages. J Mol Cell Cardiol. 2021;157:98–103. A large body of literature indicates that cardiac macrophages play an important role in heart failure. Findings from this study show that B cells modulate the phenotype of cardiac macrophage and therefore strengthen the emerging paradigm of a tight connection between B cells and heart failure.
Noutsias M, et al. Phenotypic characterization of infiltrates in dilated cardiomyopathy - diagnostic significance of T-lymphocytes and macrophages in inflammatory cardiomyopathy. Med Sci Monit. 2002;8(7):CR478–87.
Litvinukova M, et al. Cells of the adult human heart. Nature. 2020;588(7838):466–72.
Yan X, et al. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol. 2013;62:24–35.
Heinrichs M, et al. The healing myocardium mobilizes a distinct B-cell subset through a CXCL13-CXCR5-dependent mechanism. Cardiovasc Res. 2021;117(13):2664–76.
Wu L, et al. P2y12 Receptor promotes pressure overload-induced cardiac remodeling via platelet-driven inflammation in mice. Hypertension. 70(4):759–769.
Schwinger RHG. Pathophysiology of heart failure. Cardiovascular diagnosis and therapy. 2021;11(1):263–76.
Adamo L, et al. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol. 2020;17(5):269–85.
Adamo L, et al. Proteomic signatures of heart failure in relation to left ventricular ejection fraction. J Am Coll Cardiol. 2020;76(17):1982–94.
Latif N, et al. Frequency and specificity of antiheart antibodies in patients with dilated cardiomyopathy detected using SDS-PAGE and western blotting. J Am Coll Cardiol. 1993;22(5):1378–84.
Youker KA, et al. High proportion of patients with end-stage heart failure regardless of aetiology demonstrates anti-cardiac antibody deposition in failing myocardium: humoral activation, a potential contributor of disease progression. Eur Heart J. 2014;35(16):1061–8.
Keppner L, et al. Antibodies aggravate the development of ischemic heart failure. Am J Physiol Heart Circ Physiol. 2018;315(5):H1358-h1367.
Schulze K, et al. Antibodies to ADP-ATP carrier–an autoantigen in myocarditis and dilated cardiomyopathy–impair cardiac function. Circulation. 1990;81(3):959–69.
Hasham MG, et al. Systemic autoimmunity induced by the TLR7/8 agonist Resiquimod causes myocarditis and dilated cardiomyopathy in a new mouse model of autoimmune heart disease. Dis Model Mech. 2017;10(3):259–70.
Okazaki T, et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med. 2003;9(12):1477–83.
Bockstahler M, et al. Heart-specific immune responses in an animal model of autoimmune-related myocarditis mitigated by an immunoproteasome inhibitor and genetic ablation. Circulation. 2020;141(23):1885–902.
Matsui S, et al. Peptides derived from cardiovascular G-protein-coupled receptors induce morphological cardiomyopathic changes in immunized rabbits. J Mol Cell Cardiol. 1997;29(2):641–55.
Herda LR, et al. Effects of immunoadsorption and subsequent immunoglobulin G substitution on cardiopulmonary exercise capacity in patients with dilated cardiomyopathy. Am Heart J. 2010;159(5):809–16.
Staudt A, et al. Role of immunoglobulin G3 subclass in dilated cardiomyopathy: results from protein A immunoadsorption. Am Heart J. 2005;150(4):729–36.
Staudt A, et al. Potential role of autoantibodies belonging to the immunoglobulin G-3 subclass in cardiac dysfunction among patients with dilated cardiomyopathy. Circulation. 2002;106(19):2448–53.
Limas CJ, Goldenberg IF, Limas C. Autoantibodies against beta-adrenoceptors in human idiopathic dilated cardiomyopathy. Circ Res. 1989;64(1):97–103.
Jahns R, et al. Autoantibodies activating human beta1-adrenergic receptors are associated with reduced cardiac function in chronic heart failure. Circulation. 1999;99(5):649–54.
Chiale PA, et al. Differential profile and biochemical effects of antiautonomic membrane receptor antibodies in ventricular arrhythmias and sinus node dysfunction. Circulation. 2001;103(13):1765–71.
Störk S, et al. Stimulating autoantibodies directed against the cardiac beta1-adrenergic receptor predict increased mortality in idiopathic cardiomyopathy. Am Heart J. 2006;152(4):697–704.
Liu HR, et al. Relationship of myocardial remodeling to the genesis of serum autoantibodies to cardiac beta(1)-adrenoceptors and muscarinic type 2 acetylcholine receptors in rats. J Am Coll Cardiol. 2002;39(11):1866–73.
Christ T, et al. Autoantibodies against the beta1 adrenoceptor from patients with dilated cardiomyopathy prolong action potential duration and enhance contractility in isolated cardiomyocytes. J Mol Cell Cardiol. 2001;33(8):1515–25.
Iwata M, et al. Autoimmunity against the second extracellular loop of beta(1)-adrenergic receptors induces beta-adrenergic receptor desensitization and myocardial hypertrophy in vivo. Circ Res. 2001;88(6):578–86.
Jane-wit D, Altuntas CZ, Johnson JM, Yong S, Wickley PJ, Clark P, Wang Q, Popovic ZB, Penn MS, Damron DS, Perez DM. β1-Adrenergic receptor autoantibodies mediate dilated cardiomyopathy by agonistically inducing cardiomyocyte apoptosis. Circulation. 2007;116(4):399-410.
Nagatomo Y, et al. A pilot study on the role of autoantibody targeting the beta1-adrenergic receptor in the response to beta-blocker therapy for congestive heart failure. J Card Fail. 2009;15(3):224–32.
Fu LX, et al. Localization of a functional autoimmune epitope on the muscarinic acetylcholine receptor-2 in patients with idiopathic dilated cardiomyopathy. J Clin Invest. 1993;91(5):1964–8.
Stavrakis S, et al. Opposing cardiac effects of autoantibody activation of β-adrenergic and M2 muscarinic receptors in cardiac-related diseases. Int J Cardiol. 2011;148(3):331–6.
Leuschner F, et al. Absence of auto-antibodies against cardiac troponin I predicts improvement of left ventricular function after acute myocardial infarction. Eur Heart J. 2008;29(16):1949–55.
Haghikia A, et al. Evidence of autoantibodies against cardiac troponin I and sarcomeric myosin in peripartum cardiomyopathy. Basic Res Cardiol. 2015;110(6):60.
Alvarez FL, et al. Heart-specific autoantibodies induced by Coxsackievirus B3: identification of heart autoantigens. Clin Immunol Immunopathol. 1987;43(1):129–39.
Zhang Y, et al. Low level antibodies against alpha-tropomyosin are associated with increased risk of coronary heart disease. Front Pharmacol. 2020;11.
Shmilovich H, et al. Autoantibodies to cardiac troponin I in patients with idiopathic dilated and ischemic cardiomyopathy. Int J Cardiol. 2007;117(2):198–203.
Landsberger M, et al. Potential role of antibodies against cardiac Kv channel-interacting protein 2 in dilated cardiomyopathy. Am Heart J. 2008;156(1):92-99.e2.
Baba A, Yoshikawa T, Ogawa S. Autoantibodies produced against sarcolemmal Na-K-ATPase: possible upstream targets of arrhythmias and sudden death in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2002;40(6):1153–9.
Zhang M, et al. The role of natural IgM in myocardial ischemia-reperfusion injury. J Mol Cell Cardiol. 2006;41(1):62–7.
Zhang M, et al. Identification of a specific self-reactive IgM antibody that initiates intestinal ischemia/reperfusion injury. Proc Natl Acad Sci U S A. 2004;101(11):3886–91.
Vogel CW. The role of complement in myocardial infarction reperfusion injury: an underappreciated therapeutic target. Front Cell Dev Biol. 2020;8:606407.
Investigators AA, et al. Pexelizumab for acute ST-elevation myocardial infarction in patients undergoing primary percutaneous coronary intervention: a randomized controlled trial. JAMA. 2007;297(1):43–51.
Martel C, et al. Pexelizumab fails to inhibit assembly of the terminal complement complex in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Insight from a substudy of the Assessment of Pexelizumab in Acute Myocardial Infarction (APEX-AMI) trial. Am Heart J. 2012;164(1):43–51.
Ludwig RJ, et al. Mechanisms of autoantibody-induced pathology. Front Immunol. 2017;8:603.
Haudek SB, et al. Fc receptor engagement mediates differentiation of cardiac fibroblast precursor cells. Proc Natl Acad Sci U S A. 2008;105(29):10179–84.
Staudt A, et al. Fc(gamma) receptors IIa on cardiomyocytes and their potential functional relevance in dilated cardiomyopathy. J Am Coll Cardiol. 2007;49(16):1684–92.
Doing A, et al. B-cell function in chronic heart failure: antibody response to pneumococcal vaccine. J Card Fail. 2001;7(4):318–21.
Sun Y, et al. Splenic marginal zone B lymphocytes regulate cardiac remodeling after acute myocardial infarction in mice. J Am Coll Cardiol. 2022;79(7):632–47.
Mo F, et al. Are activated B cells involved in the process of myocardial fibrosis after acute myocardial infarction? An in vivo experiment. BMC Cardiovasc Disord. 2021;21(1):5.
Hanna A, Frangogiannis NG. The role of the TGF-β superfamily in myocardial infarction. Front Cardiovasc Med. 2019;6.
Li Y, et al. B cells increase myocardial inflammation by suppressing M2 macrophage polarization in Coxsackie virus B3-induced acute myocarditis. Inflammation. 2019;42(3):953–60.
Mena I, et al. The role of B lymphocytes in coxsackievirus B3 infection. Am J Pathol. 1999;155(4):1205–15.
Lu J, et al. The absence of B cells disrupts splenic and myocardial Treg homeostasis in coxsackievirus B3-induced myocarditis. Clin Exp Immunol. 2022.
Yu M, et al. TNF-α-secreting B cells contribute to myocardial fibrosis in dilated cardiomyopathy. J Clin Immunol. 2013;33(5):1002–8.
Guo Y, et al. Increased circulating interleukin 10-secreting B cells in patients with dilated cardiomyopathy. Int J Clin Exp Pathol. 2015;8(7):8107–14.
Salzer U, et al. Susceptibility to infections and adaptive immunity in adults with heart failure. ESC Heart Fail. 2022;9(2):1195–205.
Jiao J, et al. Defective circulating regulatory B cells in patients with dilated cardiomyopathy. Cell Physiol Biochem. 2018;46(1):23–35.
Goodchild TT, et al. Bone marrow-derived B cells preserve ventricular function after acute myocardial infarction. JACC Cardiovasc Interv. 2009;2(10):1005–16.
Mahrholdt H, et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114(15):1581–90.
Caforio ALP, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34(33):2636–48.
Matsumoto Y, Park IK, Kohyama K. B-cell epitope spreading is a critical step for the switch from C-protein-induced myocarditis to dilated cardiomyopathy. Am J Pathol. 2007;170(1):43–51.
Powell AM, Black MM. Epitope spreading: protection from pathogens, but propagation of autoimmunity? Clin Exp Dermatol. 2001;26(5):427–33.
Kandolf R, et al. Mechanisms and consequences of enterovirus persistence in cardiac myocytes and cells of the immune system. Virus Res. 1999;62(2):149–58.
Tschöpe C, et al. Targeting CD20+ B-lymphocytes in inflammatory dilated cardiomyopathy with rituximab improves clinical course: a case series. Eur Heart J Case Rep. 2019;3(3).
Jahns R, et al. Direct evidence for a beta 1-adrenergic receptor-directed autoimmune attack as a cause of idiopathic dilated cardiomyopathy. J Clin Invest. 2004;113(10):1419–29.
• Cordero-Reyes AM, et al. Full expression of cardiomyopathy is partly dependent on B‐cells: A pathway that involves cytokine activation, immunoglobulin deposition, and activation of apoptosis. J Am Heart Assoc. 2016;5(1):e002484. This study establishes a clear connection between B cells and myocardial hypertrophy/fibrosis and thus points to a potential role of B cells in the pathogenesis of HFpEF.
Kallikourdis M, et al. T cell costimulation blockade blunts pressure overload-induced heart failure. Nat Commun. 2017;8:14680.
van den Hoogen P, et al. Increased circulating IgG levels, myocardial immune cells and IgG deposits support a role for an immune response in pre- and end-stage heart failure. J Cell Mol Med. 2019;23(11):7505–16.
Baniaamam M, et al. Clinical improvement of cardiac function in a patient with systemic lupus erythematosus and heart failure with preserved ejection fraction treated with belimumab. BMJ Case Rep. 2021;14(1).
•• Lewis GA, et al. Pirfenidone in heart failure with preserved ejection fraction: a randomized phase 2 trial. Nat Med. 2021;27(8):1477–82. This phase II trial showed that the immunomodulatory drug pirfenidone has beneficial effects in patients with HFpEF. Since murine data connects mechanistically the cardioprotective effects of pirfenidone to its B cell modulating properties, this study points at a potential role of B cells in human HFpEF.
Aimo A, et al. Pirfenidone is a cardioprotective drug: mechanisms of action and preclinical evidence. Pharmacol Res. 2020;155:104694.
Sanchez-Trujillo L, et al. Phase II clinical trial testing the safety of a humanised monoclonal antibody anti-CD20 in patients with heart failure with reduced ejection fraction, ICFEr-RITU2: study protocol. BMJ Open. 9(3):e022826.
Zhao TX, et al. Rituximab in patients with acute ST-elevation myocardial infarction: an experimental medicine safety study. Cardiovasc Res. 2022;118(3):872–82.
Funding
Luigi Adamo is the recipient of NHLBI grants 5K08HLO145108-03 and 1R01HL160716-01.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Luigi Adamo is co-founder of i-Cordis, LLC, a start-up company focused on the development of immunomodulatory therapies for heart failure, with a special emphasis on B cells and pirfenidone derivatives. He reports a small reimbursement for 1-h consultation for Cello Health BioConsulting. He also has patents planned, issued, or pending for WO/2019/028062, WO2010121122 A3, WO2008021465 A8, and US20070249038 A1. The other authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of Topical Collection on Myocardial Disease
Rights and permissions
About this article
Cite this article
Bermea, K., Bhalodia, A., Huff, A. et al. The Role of B Cells in Cardiomyopathy and Heart Failure. Curr Cardiol Rep 24, 935–946 (2022). https://doi.org/10.1007/s11886-022-01722-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-022-01722-4