Abstract
Purpose of the Review
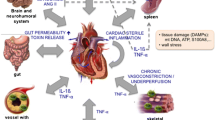
Proinflammatory cytokines are consistently elevated in congestive heart failure. In the current review, we provide an overview on the current understanding of how tumor necrosis factor-α (TNFα), a key proinflammatory cytokine, potentiates heart failure by overwhelming the anti-inflammatory responses disrupting the homeostasis.
Recent Findings
Studies have shown co-relationship between severity of heart failure and levels of the proinflammatory cytokine TNFα and one of its secondary mediators interleukin-6 (IL-6), suggesting their potential as biomarkers. Recent efforts have focused on understanding the mechanisms of how proinflammatory cytokines contribute towards cardiac dysfunction and failure. In addition, how unchecked proinflammatory cytokines and their cross-talk with sympathetic system overrides the anti-inflammatory response underlying failure.
Summary
The review offers insights on how TNFα and IL-6 contribute to cardiac dysfunction and failure. Furthermore, this provides a forum to begin the discussion on the cross-talk between sympathetic drive and proinflammatory cytokines and its determinant role in deleterious outcomes.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: •• Of major Importance
Chung MW, Tsoutsman T, Semsarian C. Hypertrophic cardiomyopathy: from gene defect to clinical disease. Cell Res. 2003;13:9–20.
Yndestad A, Damas JK, Oie E, Ueland T, Gullestad L, Aukrust P. Role of inflammation in the progression of heart failure. Curr Cardiol Rep. 2007;9:236–41.
Francis GS, Tang WH. Pathophysiology of congestive heart failure. Rev Cardiovasc Med. 2003;4(Suppl 2):S14–20.
Lefkowitz RJ, Willerson JT. Prospects for cardiovascular research. JAMA. 2001;285:581–7.
Braunwald E. Shattuck lecture--cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–9.
Valen G. Innate immunity and remodelling. Heart Fail Rev. 2011;16:71–8.
•• Mann DL. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res. 2015;116:1254–68 This article provides discussion on the mechanisms that underlie the initial response to cardiac injury and how an unchecked inflammatory response could overwhelm the anti-inflammatory resolution processes.
Gullestad L, Ueland T, Vinge LE, Finsen A, Yndestad A, Aukrust P. Inflammatory cytokines in heart failure: mediators and markers. Cardiology. 2012;122:23–35.
•• Bartekova M, Radosinska J, Jelemensky M and Dhalla NS. Role of cytokines and inflammation in heart function during health and disease. Heart Fail Rev. 2018. The review details the signlaing mechanism involved in mediating the effects of cytokines in cells with a focus on NF-κB, a key central transcirption factor that is activated in response to proinflammatory cytokines.
Ionita MG, Arslan F, de Kleijn DP, Pasterkamp G. Endogenous inflammatory molecules engage Toll-like receptors in cardiovascular disease. J Innate Immun. 2010;2:307–15.
Mann DL. The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circ Res. 2011;108:1133–45.
Libby P, Simon DI. Inflammation and thrombosis: the clot thickens. Circulation. 2001;103:1718–20.
Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109:II2–10.
Mann DL. Stress-activated cytokines and the heart: from adaptation to maladaptation. Annu Rev Physiol. 2003;65:81–101.
Ramadori G, Van Damme J, Rieder H, Meyer zum Buschenfelde KH. Interleukin 6, the third mediator of acute-phase reaction, modulates hepatic protein synthesis in human and mouse. Comparison with interleukin 1 beta and tumor necrosis factor-alpha. Eur J Immunol. 1988;18:1259–64.
•• Mohan ML, Vasudevan NT, Naga Prasad SV. Proinflammatory cytokines mediate GPCR dysfunction. J Cardiovasc Pharmacol. 2017;70:61–73 This manusript provides in-depth discussion on the cross-talk between the proinflammatory cytokines and G-protein coupled receptors describing how these cytokines inhibit receptor function.
Mommersteeg PM, Kupper N, Schoormans D, Emons W, Pedersen SS. Health-related quality of life is related to cytokine levels at 12 months in patients with chronic heart failure. Brain Behav Immun. 2010;24:615–22.
Hartupee J, Mann DL. Positioning of inflammatory biomarkers in the heart failure landscape. J Cardiovasc Transl Res. 2013;6:485–92.
Ueland T, Gullestad L, Nymo SH, Yndestad A, Aukrust P, Askevold ET. Inflammatory cytokines as biomarkers in heart failure. Clin Chim Acta. 2015;443:71–7.
Divakaran VG, Evans S, Topkara VK, Diwan A, Burchfield J, Gao F, et al. Tumor necrosis factor receptor-associated factor 2 signaling provokes adverse cardiac remodeling in the adult Mammalian heart. Circulation Heart Failure. 2013;6:535–43.
Garlie JB, Hamid T, Gu Y, Ismahil MA, Chandrasekar B, Prabhu SD. Tumor necrosis factor receptor 2 signaling limits beta-adrenergic receptor-mediated cardiac hypertrophy in vivo. Basic Research in Cardiology. 2011;106:1193–205.
Vasudevan NT, Mohan ML, Gupta MK, Martelli EE, Hussain AK, Qin Y, et al. Gbetagamma-independent recruitment of G-protein coupled receptor kinase 2 drives tumor necrosis factor alpha-induced cardiac beta-adrenergic receptor dysfunction. Circulation. 2013;128:377–87.
Prabhu SD. Cytokine-induced modulation of cardiac function. Circulation research. 2004;95:1140–53.
Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–12.
Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11:395–402.
Mezzaroma E, Toldo S, Farkas D, Seropian IM, Van Tassell BW, Salloum FN, et al. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci U S A. 2011;108:19725–30.
Matzinger P. Friendly and dangerous signals: is the tissue in control? Nat Immunol. 2007;8:11–3.
Mann DL, Topkara VK, Evans S, Barger PM. Innate immunity in the adult mammalian heart: for whom the cell tolls. Trans Am Clin Climatol Assoc. 2010;121:34–50 discussion 50–1.
Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–5.
Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol. 2010;72:19–44.
Gottlieb RA, Carreira RS. Autophagy in health and disease. 5. Mitophagy as a way of life. Am J Physiol Cell Physiol. 2010;299:C203–10.
Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511.
Yan ZQ, Hansson GK. Innate immunity, macrophage activation, and atherosclerosis. Immunol Rev. 2007;219:187–203.
Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–20.
Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev. 2009;227:221–33.
Erickson B, Sperber K, Frishman WH. Toll-like receptors: new therapeutic targets for the treatment of atherosclerosis, acute coronary syndromes, and myocardial failure. Cardiol Rev. 2008;16:273–9.
Frantz S, Ertl G, Bauersachs J. Mechanisms of disease: Toll-like receptors in cardiovascular disease. Nat Clin Pract Cardiovasc Med. 2007;4:444–54.
Geddes K, Magalhaes JG, Girardin SE. Unleashing the therapeutic potential of NOD-like receptors. Nat Rev Drug Discov. 2009;8:465–79.
Kawaguchi M, Takahashi M, Hata T, Kashima Y, Usui F, Morimoto H, et al. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123:594–604.
Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human toll-like receptors and related genes. Biol Pharm Bull. 2005;28:886–92.
Lech M, Susanti HE, Rommele C, Grobmayr R, Gunthner R, Anders HJ. Quantitative expression of C-type lectin receptors in humans and mice. Int J Mol Sci. 2012;13:10113–31.
Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–21.
Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–35.
Fildes JE, Shaw SM, Yonan N, Williams SG. The immune system and chronic heart failure: is the heart in control? J Am Coll Cardiol. 2009;53:1013–20.
Birks EJ, Latif N, Owen V, Bowles C, Felkin LE, Mullen AJ, et al. Quantitative myocardial cytokine expression and activation of the apoptotic pathway in patients who require left ventricular assist devices. Circulation. 2001;104:I233–40.
Tang H, Zhong Y, Zhu Y, Zhao F, Cui X, Wang Z. Low responder T cell susceptibility to the suppressive function of regulatory T cells in patients with dilated cardiomyopathy. Heart. 2010;96:765–71.
Tang TT, Ding YJ, Liao YH, Yu X, Xiao H, Xie JJ, et al. Defective circulating CD4CD25+Foxp3+CD127(low) regulatory T-cells in patients with chronic heart failure. Cell Physiol Biochem. 2010;25:451–8.
Kvakan H, Kleinewietfeld M, Qadri F, Park JK, Fischer R, Schwarz I, et al. Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation. 2009;119:2904–12.
Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT, Anti TNFTACHFI. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107:3133–40.
Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation. 2004;109:1594–602.
Mann DL. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res. 2002;91:988–98.
Lawrence T, Fong C. The resolution of inflammation: anti-inflammatory roles for NF-kappaB. Int J Biochem Cell Biol. 2010;42:519–23.
Hall G, Hasday JD, Rogers TB. Regulating the regulator: NF-kappaB signaling in heart. J Mol Cell Cardiol. 2006;41:580–91.
Jones WK, Brown M, Wilhide M, He S, Ren X. NF-kappaB in cardiovascular disease: diverse and specific effects of a “general” transcription factor? Cardiovasc Toxicol. 2005;5:183–202.
Li C, Browder W, Kao RL. Early activation of transcription factor NF-kappaB during ischemia in perfused rat heart. Am J Physiol. 1999;276:H543–52.
Li C, Kao RL, Ha T, Kelley J, Browder IW, Williams DL. Early activation of IKKbeta during in vivo myocardial ischemia. Am J Physiol Heart Circ Physiol. 2001;280:H1264–71.
Sun SC. The noncanonical NF-kappaB pathway. Immunol Rev. 2012;246:125–40.
Gordon JW, Shaw JA, Kirshenbaum LA. Multiple facets of NF-kappaB in the heart: to be or not to NF-kappaB. Circ Res. 2011;108:1122–32.
Seta Y, Shan K, Bozkurt B, Oral H, Mann DL. Basic mechanisms in heart failure: the cytokine hypothesis. J Card Fail. 1996;2:243–9.
Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res. 2016;119:91–112.
Massion PB, Feron O, Dessy C, Balligand JL. Nitric oxide and cardiac function: ten years after, and continuing. Circ Res. 2003;93:388–98.
Prabhu SD. Nitric oxide protects against pathological ventricular remodeling: reconsideration of the role of NO in the failing heart. Circ Res. 2004;94:1155–7.
Kojda G, Kottenberg K. Regulation of basal myocardial function by NO. Cardiovasc Res. 1999;41:514–23.
Finkel MS, Oddis CV, Jacob TD, Watkins SC, Hattler BG, Simmons RL. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science. 1992;257:387–9.
McGowan FX Jr, Takeuchi K, del Nido PJ, Davis PJ, Lancaster JR Jr, Hattler BG. Myocardial effects of interleukin-2. Transplant Proc. 1994;26:209–10.
Kinugawa K, Takahashi T, Kohmoto O, Yao A, Aoyagi T, Momomura S, et al. Nitric oxide-mediated effects of interleukin-6 on [Ca2+]i and cell contraction in cultured chick ventricular myocytes. Circ Res. 1994;75:285–95.
Sugishita K, Kinugawa K, Shimizu T, Harada K, Matsui H, Takahashi T, et al. Cellular basis for the acute inhibitory effects of IL-6 and TNF- alpha on excitation-contraction coupling. J Mol Cell Cardiol. 1999;31:1457–67.
Goldhaber JI, Kim KH, Natterson PD, Lawrence T, Yang P, Weiss JN. Effects of TNF-alpha on [Ca2+]i and contractility in isolated adult rabbit ventricular myocytes. Am J Physiol. 1996;271:H1449–55.
Alloatti G, Penna C, De Martino A, Montrucchio G, Camussi G. Role of nitric oxide and platelet-activating factor in cardiac alterations induced by tumor necrosis factor-alpha in the guinea-pig papillary muscle. Cardiovasc Res. 1999;41:611–9.
Hofmann U, Domeier E, Frantz S, Laser M, Weckler B, Kuhlencordt P, et al. Increased myocardial oxygen consumption by TNF-alpha is mediated by a sphingosine signaling pathway. Am J Physiol Heart Circ Physiol. 2003;284:H2100–5.
Panas D, Khadour FH, Szabo C, Schulz R. Proinflammatory cytokines depress cardiac efficiency by a nitric oxide-dependent mechanism. Am J Physiol. 1998;275:H1016–23.
Cain BS, Meldrum DR, Dinarello CA, Meng X, Joo KS, Banerjee A, et al. Tumor necrosis factor-alpha and interleukin-1beta synergistically depress human myocardial function. Crit Care Med. 1999;27:1309–18.
Kumar A, Brar R, Wang P, Dee L, Skorupa G, Khadour F, et al. Role of nitric oxide and cGMP in human septic serum-induced depression of cardiac myocyte contractility. Am J Physiol. 1999;276:R265–76.
Bartel S, Stein B, Eschenhagen T, Mende U, Neumann J, Schmitz W, et al. Protein phosphorylation in isolated trabeculae from nonfailing and failing human hearts. Mol Cell Biochem. 1996;157:171–9.
Grandel U, Fink L, Blum A, Heep M, Buerke M, Kraemer HJ, et al. Endotoxin-induced myocardial tumor necrosis factor-alpha synthesis depresses contractility of isolated rat hearts: evidence for a role of sphingosine and cyclooxygenase-2-derived thromboxane production. Circulation. 2000;102:2758–64.
Yokoyama T, Vaca L, Rossen RD, Durante W, Hazarika P, Mann DL. Cellular basis for the negative inotropic effects of tumor necrosis factor-alpha in the adult mammalian heart. J Clin Invest. 1993;92:2303–12.
Edmunds NJ, Lal H, Woodward B. Effects of tumour necrosis factor-alpha on left ventricular function in the rat isolated perfused heart: possible mechanisms for a decline in cardiac function. Br J Pharmacol. 1999;126:189–96.
Oral H, Dorn GW 2nd, Mann DL. Sphingosine mediates the immediate negative inotropic effects of tumor necrosis factor-alpha in the adult mammalian cardiac myocyte. J Biol Chem. 1997;272:4836–42.
Kolesnick R. The therapeutic potential of modulating the ceramide/sphingomyelin pathway. J Clin Invest. 2002;110:3–8.
Liu B, Andrieu-Abadie N, Levade T, Zhang P, Obeid LM, Hannun YA. Glutathione regulation of neutral sphingomyelinase in tumor necrosis factor-alpha-induced cell death. J Biol Chem. 1998;273:11313–20.
Jayadev S, Linardic CM, Hannun YA. Identification of arachidonic acid as a mediator of sphingomyelin hydrolysis in response to tumor necrosis factor alpha. J Biol Chem. 1994;269:5757–63.
Friedrichs GS, Swillo RE, Jow B, Bridal T, Numann R, Warner LM, et al. Sphingosine modulates myocyte electrophysiology, induces negative inotropy, and decreases survival after myocardial ischemia. J Cardiovasc Pharmacol. 2002;39:18–28.
Dettbarn CA, Betto R, Salviati G, Palade P, Jenkins GM, Sabbadini RA. Modulation of cardiac sarcoplasmic reticulum ryanodine receptor by sphingosine. J Mol Cell Cardiol. 1994;26:229–42.
Schreur KD, Liu S. Involvement of ceramide in inhibitory effect of IL-1 beta on L-type Ca2+ current in adult rat ventricular myocytes. Am J Phys. 1997;272:H2591–8.
Torre-Amione G, Kapadia S, Lee J, Bies RD, Lebovitz R, Mann DL. Expression and functional significance of tumor necrosis factor receptors in human myocardium. Circulation. 1995;92:1487–93.
Stamm C, Cowan DB, Friehs I, Noria S, del Nido PJ, McGowan FX Jr. Rapid endotoxin-induced alterations in myocardial calcium handling: obligatory role of cardiac TNF-alpha. Anesthesiology. 2001;95:1396–405.
Edmunds NJ, Woodward B. Effects of tumour necrosis factor-alpha on the coronary circulation of the rat isolated perfused heart: a potential role for thromboxane A2 and sphingosine. Br J Pharmacol. 1998;124:493–8.
Stamm C, Friehs I, Cowan DB, Moran AM, Cao-Danh H, Duebener LF, et al. Inhibition of tumor necrosis factor-alpha improves postischemic recovery of hypertrophied hearts. Circulation. 2001;104:I350–5.
Cailleret M, Amadou A, Andrieu-Abadie N, Nawrocki A, Adamy C, Ait-Mamar B, et al. N-acetylcysteine prevents the deleterious effect of tumor necrosis factor-(alpha) on calcium transients and contraction in adult rat cardiomyocytes. Circulation. 2004;109:406–11.
Robinson BS, Hii CS, Poulos A, Ferrante A. Activation of neutral sphingomyelinase in human neutrophils by polyunsaturated fatty acids. Immunology. 1997;91:274–80.
Mazurais D, Robert P, Gout B, Berrebi-Bertrand I, Laville MP, Calmels T. Cell type-specific localization of human cardiac S1P receptors. J Histochem Cytochem. 2002;50:661–70.
Morales-Ruiz M, Lee MJ, Zollner S, Gratton JP, Scotland R, Shiojima I, et al. Sphingosine 1-phosphate activates Akt, nitric oxide production, and chemotaxis through a Gi protein/phosphoinositide 3-kinase pathway in endothelial cells. J Biol Chem. 2001;276:19672–7.
Amadou A, Nawrocki A, Best-Belpomme M, Pavoine C, Pecker F. Arachidonic acid mediates dual effect of TNF-alpha on Ca2+ transients and contraction of adult rat cardiomyocytes. Am J Physiol Cell Physiol. 2002;282:C1339–47.
Liu SJ, McHowat J. Stimulation of different phospholipase A2 isoforms by TNF-alpha and IL-1beta in adult rat ventricular myocytes. Am J Physiol. 1998;275:H1462–72.
Damron DS, Summers BA. Arachidonic acid enhances contraction and intracellular Ca2+ transients in individual rat ventricular myocytes. Am J Physiol. 1997;272:H350–9.
de Bracco MM, Fink SB, Finiasz MR, Borda ES, Sterin-Borda L. Positive inotropic effect of interleukin-2. Role of phospholipases and protein kinase C. Int J Immunopharmacol. 1991;13:509–15.
Fink SB, Finiasz M, Sterin-Borda L, Borda E, de Bracco MM. Stimulation of heart contractility by supernatants from lectin-activated lymphocytes. Role of IL-2. Int J Immunopharmacol. 1989;11:367–70.
Kang JX, Leaf A. Effects of long-chain polyunsaturated fatty acids on the contraction of neonatal rat cardiac myocytes. Proc Natl Acad Sci U S A. 1994;91:9886–90.
Pavoine C, Magne S, Sauvadet A, Pecker F. Evidence for a beta2-adrenergic/arachidonic acid pathway in ventricular cardiomyocytes. Regulation by the beta1-adrenergic/camp pathway. J Biol Chem. 1999;274:628–37.
Kumar A, Kosuri R, Kandula P, Dimou C, Allen J, Parrillo JE. Effects of epinephrine and amrinone on contractility and cyclic adenosine monophosphate generation of tumor necrosis factor alpha-exposed cardiac myocytes. Crit Care Med. 1999;27:286–92.
Liu SJ, Zhou W, Kennedy RH. Suppression of beta-adrenergic responsiveness of L-type Ca2+ current by IL-1beta in rat ventricular myocytes. Am J Physiol. 1999;276:H141–8.
De Paepe B, Creus KK, De Bleecker JL. The tumor necrosis factor superfamily of cytokines in the inflammatory myopathies: potential targets for therapy. Clin Dev Immunol. 2012;2012:369432.
Yndestad A, Damas JK, Geir Eiken H, Holm T, Haug T, Simonsen S, et al. Increased gene expression of tumor necrosis factor superfamily ligands in peripheral blood mononuclear cells during chronic heart failure. Cardiovasc Res. 2002;54:175–82.
Loncar G, Bozic B, Cvorovic V, Radojicic Z, Dimkovic S, Markovic N, et al. Relationship between RANKL and neuroendocrine activation in elderly males with heart failure. Endocrine. 2010;37:148–56.
Burchfield JS, Dong JW, Sakata Y, Gao F, Tzeng HP, Topkara VK, et al. The cytoprotective effects of tumor necrosis factor are conveyed through tumor necrosis factor receptor-associated factor 2 in the heart. Circ Heart Fail. 2010;3:157–64.
Cabal-Hierro L, Lazo PS. Signal transduction by tumor necrosis factor receptors. Cell Signal. 2012;24:1297–305.
Al-Lamki RS, Wang J, Vandenabeele P, Bradley JA, Thiru S, Luo D, et al. TNFR1- and TNFR2-mediated signaling pathways in human kidney are cell type-specific and differentially contribute to renal injury. FASEB J. 2005;19:1637–45.
Faustman DL, Davis M. TNF Receptor 2 and Disease: Autoimmunity and Regenerative Medicine. Front Immunol. 2013;4:478.
Torre-Amione G, Kapadia S, Lee J, Durand JB, Bies RD, Young JB, et al. Tumor necrosis factor-alpha and tumor necrosis factor receptors in the failing human heart. Circulation. 1996;93:704–11.
Dibbs ZI, Diwan A, Nemoto S, DeFreitas G, Abdellatif M, Carabello BA, et al. Targeted overexpression of transmembrane tumor necrosis factor provokes a concentric cardiac hypertrophic phenotype. Circulation. 2003;108:1002–8.
Diwan A, Dibbs Z, Nemoto S, DeFreitas G, Carabello BA, Sivasubramanian N, et al. Targeted overexpression of noncleavable and secreted forms of tumor necrosis factor provokes disparate cardiac phenotypes. Circulation. 2004;109:262–8.
Li X, Moody MR, Engel D, Walker S, Clubb FJ Jr, Sivasubramanian N, et al. Cardiac-specific overexpression of tumor necrosis factor-alpha causes oxidative stress and contractile dysfunction in mouse diaphragm. Circulation. 2000;102:1690–6.
Kubota T, McTiernan CF, Frye CS, Slawson SE, Lemster BH, Koretsky AP, et al. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha. Circ Res. 1997;81:627–35.
Gulick T, Chung MK, Pieper SJ, Lange LG, Schreiner GF. Interleukin 1 and tumor necrosis factor inhibit cardiac myocyte beta-adrenergic responsiveness. Proc Natl Acad Sci U S A. 1989;86:6753–7.
Chung MK, Gulick TS, Rotondo RE, Schreiner GF, Lange LG. Mechanism of cytokine inhibition of beta-adrenergic agonist stimulation of cyclic AMP in rat cardiac myocytes. Impairment of signal transduction. Circ Res. 1990;67:753–63.
Muller-Werdan U, Schumann H, Fuchs R, Reithmann C, Loppnow H, Koch S, et al. Tumor necrosis factor alpha (TNF alpha) is cardiodepressant in pathophysiologically relevant concentrations without inducing inducible nitric oxide-(NO)-synthase (iNOS) or triggering serious cytotoxicity. J Mol Cell Cardiol. 1997;29:2915–23.
Tan KS, Nackley AG, Satterfield K, Maixner W, Diatchenko L, Flood PM. Beta2 adrenergic receptor activation stimulates pro-inflammatory cytokine production in macrophages via PKA- and NF-kappaB-independent mechanisms. Cell Signal. 2007;19:251–60.
Verhoeckx KC, Doornbos RP, Witkamp RF, van der Greef J, Rodenburg RJ. Beta-adrenergic receptor agonists induce the release of granulocyte chemotactic protein-2, oncostatin M, and vascular endothelial growth factor from macrophages. Int Immunopharmacol. 2006;6:1–7.
Kim MH, Gorouhi F, Ramirez S, Granick JL, Byrne BA, Soulika AM, et al. Catecholamine stress alters neutrophil trafficking and impairs wound healing by beta2-adrenergic receptor-mediated upregulation of IL-6. J Investig Dermatol. 2014;134:809–17.
Roth Flach RJ, Matevossian A, Akie TE, Negrin KA, Paul MT, Czech MP. beta3-Adrenergic receptor stimulation induces E-selectin-mediated adipose tissue inflammation. J Biol Chem. 2013;288:2882–92.
Prabhu SD, Chandrasekar B, Murray DR, Freeman GL. beta-adrenergic blockade in developing heart failure: effects on myocardial inflammatory cytokines, nitric oxide, and remodeling. Circulation. 2000;101:2103–9.
Chandrasekar B, Marelli-Berg FM, Tone M, Bysani S, Prabhu SD, Murray DR. Beta-adrenergic stimulation induces interleukin-18 expression via beta2-AR, PI3K, Akt, IKK, and NF-kappaB. Biochem Biophys Res Commun. 2004;319:304–11.
Chandrasekar B, Vemula K, Surabhi RM, Li-Weber M, Owen-Schaub LB, Jensen LE, et al. Activation of intrinsic and extrinsic proapoptotic signaling pathways in interleukin-18-mediated human cardiac endothelial cell death. J Biol Chem. 2004;279:20221–33.
Grisanti LA, Repas AA, Talarico JA, Gold JI, Carter RL, Koch WJ, et al. Temporal and gefitinib-sensitive regulation of cardiac cytokine expression via chronic beta-adrenergic receptor stimulation. Am J Physiol Heart Circ Physiol. 2015;308:H316–30.
Pera T, Penn RB. Bronchoprotection and bronchorelaxation in asthma: New targets, and new ways to target the old ones. Pharmacol Ther. 2016;164:82–96.
Koch WJ, Lefkowitz RJ, Rockman HA. Functional consequences of altering myocardial adrenergic receptor signaling. Annu Rev Physiol. 2000;62:237–60.
Koch WJ, Rockman HA, Samama P, Hamilton RA, Bond RA, Milano CA, et al. Cardiac function in mice overexpressing the beta-adrenergic receptor kinase or a beta ARK inhibitor. Science. 1995;268:1350–3.
Lefkowitz RJ. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J Biol Chem. 1998;273:18677–80.
Garcia-Guerra L, Nieto-Vazquez I, Vila-Bedmar R, Jurado-Pueyo M, Zalba G, Diez J, et al. G protein-coupled receptor kinase 2 plays a relevant role in insulin resistance and obesity. Diabetes. 2010;59:2407–17.
Lefkowitz RJ, Rockman HA, Koch WJ. Catecholamines, cardiac beta-adrenergic receptors, and heart failure. Circulation. 2000;101:1634–7.
White DC, Hata JA, Shah AS, Glower DD, Lefkowitz RJ, Koch WJ. Preservation of myocardial beta-adrenergic receptor signaling delays the development of heart failure after myocardial infarction. Proc Natl Acad Sci U S A. 2000;97:5428–33.
Hamid T, Gu Y, Ortines RV, Bhattacharya C, Wang G, Xuan YT, et al. Divergent tumor necrosis factor receptor-related remodeling responses in heart failure: role of nuclear factor-kappaB and inflammatory activation. Circulation. 2009;119:1386–97.
Kalliolias GD, Ivashkiv LB. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol. 2016;12:49–62.
Hotamisligil GS. The role of TNFalpha and TNF receptors in obesity and insulin resistance. J Intern Med. 1999;245:621–5.
Felten SY, Madden KS, Bellinger DL, Kruszewska B, Moynihan JA, Felten DL. The role of the sympathetic nervous system in the modulation of immune responses. Adv Pharmacol. 1998;42:583–7.
Felten DL, Felten SY, Bellinger DL, Lorton D. Noradrenergic and peptidergic innervation of secondary lymphoid organs: role in experimental rheumatoid arthritis. Eur J Clin Invest. 1992;22(Suppl 1):37–41.
Felten SY, Felten DL, Bellinger DL, Olschowka JA. Noradrenergic and peptidergic innervation of lymphoid organs. Chem Immunol. 1992;52:25–48.
Friedman EM, Irwin MR. Modulation of immune cell function by the autonomic nervous system. Pharmacol Ther. 1997;74:27–38.
Bellinger DL, Millar BA, Perez S, Carter J, Wood C, ThyagaRajan S, et al. Sympathetic modulation of immunity: relevance to disease. Cell Immunol. 2008;252:27–56.
Eskandari F, Sternberg EM. Neural-immune interactions in health and disease. Ann N Y Acad Sci. 2002;966:20–7.
Hatfield SM, Petersen BH, DiMicco JA. Beta-adrenergic agonists blocked the expression of IL-2 receptors on mitogen-stimulated lymphocytes and IL-2-dependent T cell lines. J Immunol. 1988;141:1418–20.
Oberbeck R. Catecholamines: physiological immunomodulators during health and illness. Curr Med Chem. 2006;13:1979–89.
Stevens-Felten SY, Bellinger DL. Noradrenergic and peptidergic innervation of lymphoid organs. Chem Immunol. 1997;69:99–131.
Heijnen CJ, Kavelaars A. The importance of being receptive. J Neuroimmunol. 1999;100:197–202.
Kin NW, Sanders VM. It takes nerve to tell T and B cells what to do. J Leukoc Biol. 2006;79:1093–104.
Sitkauskiene B, Sakalauskas R. The role of beta(2)-adrenergic receptors in inflammation and allergy. Curr Drug Targets Inflamm Allergy. 2005;4:157–62.
Vasudevan NT, Mohan ML, Goswami SK, Naga Prasad SV. Regulation of beta-adrenergic receptor function: an emphasis on receptor resensitization. Cell Cycle. 2011;10:3684–91.
Madden KS, Felten SY, Felten DL, Bellinger DL. Sympathetic nervous system--immune system interactions in young and old Fischer 344 rats. Ann N Y Acad Sci. 1995;771:523–34.
Madden KS, Felten DL. Experimental basis for neural-immune interactions. Physiol Rev. 1995;75:77–106.
Madden KS, Sanders VM, Felten DL. Catecholamine influences and sympathetic neural modulation of immune responsiveness. Annu Rev Pharmacol Toxicol. 1995;35:417–48.
Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007). Brain Behav Immun. 2007;21:736–45.
Link AA, Kino T, Worth JA, JL MG, Crane ML, Chrousos GP, et al. Ligand-activation of the adenosine A2a receptors inhibits IL-12 production by human monocytes. J Immunol. 2000;164:436–42.
Benarroch EE. Autonomic-mediated immunomodulation and potential clinical relevance. Neurology. 2009;73:236–42.
Elenkov I. Neuroendocrine effects on immune system. In: L. J. De Groot, G. Chrousos, K. Dungan, K. R. Feingold, A. Grossman, J. M. Hershman, C. Koch, M. Korbonits, R. McLachlan, M. New, J. Purnell, R. Rebar, F. Singer and A. Vinik, eds. Endotext South Dartmouth (MA); 2000.
Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638.
Imrich R, Tibenska E, Koska J, Ksinantova L, Kvetnansky R, Bergendiova-Sedlackova K, et al. Repeated stress-induced stimulation of catecholamine response is not followed by altered immune cell redistribution. Ann N Y Acad Sci. 2004;1018:266–72.
Zhang X, Hartung JE, Bortsov AV, Kim S, O'Buckley SC, Kozlowski J, et al. Sustained stimulation of beta2- and beta3-adrenergic receptors leads to persistent functional pain and neuroinflammation. Brain Behav Immun. 2018.
Bierhaus A, Humpert PM, Nawroth PP. Linking stress to inflammation. Anesthesiol Clin. 2006;24:325–40.
Sanders VM, Straub RH. Norepinephrine, the beta-adrenergic receptor, and immunity. Brain Behav Immun. 2002;16:290–332.
Kiecolt-Glaser JK, Glaser R. Depression and immune function: central pathways to morbidity and mortality. J Psychosom Res. 2002;53:873–6.
Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Psychoneuroimmunology: psychological influences on immune function and health. J Consult Clin Psychol. 2002;70:537–47.
Loudon RP, Perussia B, Benovic JL. Differentially regulated expression of the G-protein-coupled receptor kinases, betaARK and GRK6, during myelomonocytic cell development in vitro. Blood. 1996;88:4547–57.
Lombardi MS, Kavelaars A, Schedlowski M, Bijlsma JW, Okihara KL, Van de Pol M, et al. Decreased expression and activity of G-protein-coupled receptor kinases in peripheral blood mononuclear cells of patients with rheumatoid arthritis. FASEB J. 1999;13:715–25.
Vroon A, Kavelaars A, Limmroth V, Lombardi MS, Goebel MU, Van Dam AM, et al. G protein-coupled receptor kinase 2 in multiple sclerosis and experimental autoimmune encephalomyelitis. J Immunol. 2005;174:4400–6.
Hata JA, Williams ML, Schroder JN, Lima B, Keys JR, Blaxall BC, et al. Lymphocyte levels of GRK2 (betaARK1) mirror changes in the LVAD-supported failing human heart: lower GRK2 associated with improved beta-adrenergic signaling after mechanical unloading. J Card Fail. 2006;12:360–8.
Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–33.
Hsu H, Shu HB, Pan MG, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308.
Brenner D, Blaser H, Mak TW. Regulation of tumour necrosis factor signalling: live or let die. Nat Rev Immunol. 2015;15:362–74.
Bianchi K, Meier P. A tangled web of ubiquitin chains: breaking news in TNF-R1 signaling. Mol Cell. 2009;36:736–42.
Al-Lamki RS, Brookes AP, Wang J, Reid MJ, Parameshwar J, Goddard MJ, et al. TNF receptors differentially signal and are differentially expressed and regulated in the human heart. Am J Transpl. 2009;9:2679–96.
Grell M, Douni E, Wajant H, Lohden M, Clauss M, Maxeiner B, et al. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell. 1995;83:793–802.
Sun M, Dawood F, Wen WH, Chen M, Dixon I, Kirshenbaum LA, et al. Excessive tumor necrosis factor activation after infarction contributes to susceptibility of myocardial rupture and left ventricular dysfunction. Circulation. 2004;110:3221–8.
Funding
The work in part is supported by NIH ROO HL132882 and Cleveland Clinic Startup funds (S.M.S) and by NIH RO1 HL089473 and HL128382 (S.V.NP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Sarah M. Schumacher and Sathyamangla V. Naga Prasad declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Myocardial Disease
Rights and permissions
About this article
Cite this article
Schumacher, S.M., Naga Prasad, S.V. Tumor Necrosis Factor-α in Heart Failure: an Updated Review. Curr Cardiol Rep 20, 117 (2018). https://doi.org/10.1007/s11886-018-1067-7
Published:
DOI: https://doi.org/10.1007/s11886-018-1067-7