Abstract
Alzheimer’s disease (AD) and Parkinson’s disease (PD) are important neurodegenerative disorders, especially in an aging population context that prevails in high-developed countries and Europe in particular. It is known that exposure to particulate matter (PM) leads to the production and deposition of aggregate clusters of proteins, which are linked to neurological disorders and impediments. Nonetheless, only a few works study the short-term exposure to PM and its association with hospital admissions or mortality due to AD or PD. This study assesses the association between exposure to PM and emergency hospital admissions for AD and PD in an aging metropole, serving as a case study for most European big cities. Daily emergency hospital admissions due to AD and PD data were obtained for the 2012 to 2015 period and multivariate Poisson regression models were used to evaluate the association between PM and admissions while controlling for the day of the week, seasonality, and environmental factors. Furthermore, lagged observations were assessed. Results show that an increase in exposure to PM2.5 resulted in a percentage increase in emergency hospital admissions due to AD and PD. Also, age was an effect modifier for PD admissions. Additionally, greater effects were felt at shorter lags for AD and delayed/longer lags for PD. This study found a relationship between short-term exposure to PM and AD and PD hospital admissions in an urban context, drawing attention to the importance of air pollution for urban health, especially in areas with an aged population structure.
Similar content being viewed by others
Background
Alzheimer’s disease (AD) and Parkinson’s disease (PD) are important neurodegenerative disorders, particularly in a context of an aging population, as is the case in high-developed countries, especially in Europe (Pringsheim et al. 2014).
The impacts of air pollutants concentration on mortality and hospital admissions have been consistently proved throughout the world, where the highlight is set on the importance of respiratory and cardiac diseases (Aturinde et al. 2021; He et al. 2021; Jin et al. 2022; Karbakhsh et al. 2022; Peng et al. 2022; Rodríguez et al. 2022.; Tarín-Carrasco et al. 2021; Yee et al. 2021; Zhang et al. 2020). But, over the last two decades, exposure to air pollutants has been associated with exacerbated cognitive dysfunction and enhanced progression of neurodegenerative processes underlying AD and PD, being advocated as a risk enhancer for hospital admissions or mortality due to these diseases (Babadjouni et al. 2017; Block and Calderón-Garcidueñas 2009; Calderón-Garcidueñas et al. 2002, 2004; Donaldson et al. 2005; Hu et al. 2019; Kasdagli et al. 2019; Kilian and Kitazawa 2018; Liu et al. 2016; Livingston et al. 2020; Mir et al. 2020; Moulton and Yang 2012; Peters et al. 2019; Schikowski and Altuğ 2020; Shou et al. 2019).
It is known that exposure to particulate matter (PM) leads to the production and deposition of aggregated clusters of proteins, which in turn are closely linked to neurological disorders and impairments (Block and Calderón-Garcidueñas 2009; Calderón-Garcidueñas et al. 2020). In fact, many studies regarding neurological disorders tend to focus on “fine particles” which are 2.5 μm in diameter and smaller (PM2.5). This happens because these particles not only damage human airways when entering in the organism but are also able to penetrate the bloodstream and cross the blood–brain barrier, thus reaching vital organs and entering in the central nervous system (CNS) (Power et al. 2016). When this occurs, PM2.5 can induce oxidative stress and brain inflammation and damage DNA, leading to serious complications in the human brain (Boda et al. 2020; Calderón-Garcidueñas et al. 2004, 2019; Hahad et al. 2020; Mallhi et al. 2021; Power et al. 2016). Ultimately, exposure to PM2.5 may cause neurological diseases, including Alzheimer’s disease, Parkinson’s disease, and other forms of dementia (Bandyopadhyay 2016).
Thus, PM2.5 is expected to be the most researched pollutant and the one that usually demonstrates more connections with neurodegenerative disorders, particularly AD and PD (Culqui et al. 2017; Fu et al. 2019; Kioumourtzoglou et al. 2016; Nunez et al. 2021; Wang et al. 2020; Younan et al. 2020; Yu et al. 2021; Zanobetti et al. 2014). Although these studies identify an increased risk of AD and PD episodes due to PM2.5 concentrations, more studies in different territories and with different methodologies and approaches are needed. Going through short-term exposure studies, in which has been suggested that high levels of PM pollution affect short-term cognition Shehab and Pope (2019), and as Lee et al. (2017) and Zanobetti et al. (2014) pointed out, short-term concentrations can also affect the progression of neurological disease. So, further analysis is a clear need, especially for the European framework, where urban populations still suffer from high levels of PM (Sicard et al. 2021).
The relation between pollution and neurodegenerative disorders gains importance in an urban context, where PM assumes higher values due to the concentration of economic activities and mobility in a private car. This is even more significant when a large part of the vehicle fleet runs on diesel engines, as it happens in Europe, due to the fact that gases from diesel exhaust represent an important component of air pollution (Ghio et al. 2012), enhancing air pollution because the private vehicle is used as an enabler for the individual’s daily activities and practices, as is Lisbon case (Franco and Marques da Costa 2021; Louro et al. 2021a). In fact, some studies have analyzed the proximity to major traffic lanes and related pollution, to which PM is linked (Woodward et al. 2015), and found higher risks of AD and PD (Cacciottolo et al. 2020; Chen et al. 2017; H.-R. Yu et al. 2020; Yuchi et al. 2020), reinforcing the relevance of combating pollution in the planning for healthier and more livable cities (Khorrami et al. 2020; Louro et al. 2019; Louro et al. 2021b; Vujcic et al. 2019), even more in a post-pandemic situation (Attademo and Bernardini 2017; Gunn et al. 2017; Zhang and Gu 2013).
In Portugal, few recent epidemiologic studies regarding Alzheimer’s disease (AD) and Parkinson’s disease (PD) estimate to exist between 80,144 and 112,201 cases of AD (Santana et al. 2015), and about 180 cases per 100,000 inhabitants (CI 95% 30–327 per 100,000) (Ferreira et al. 2017). The important influence of multiple air pollutants on admissions and/or mortality due to a wide range of diseases has recently been studied in the Portuguese territory (Brito et al. 2021; Camacho et al. 2020; Franco et al. 2020; Martins et al. 2021; Rodrigues et al. 2021). Although a few time series studies addressing the relationship between air pollutants and AD and PD, related to hospital admissions or mortality, have been carried out in other countries, in Portugal, there is none. This gap was identified; thus, the present work is the first ecological time series study to address the effects of air pollutants on emergency hospital admissions on AD and PD in Portugal and the first to analyze both diseases in Europe.
Therefore, this study is of the uttermost importance for Portugal, its big cities and metropolitan areas, as nothing has yet been done regarding exposure to air pollutants and AD or PD. But it is also important for European metropolitan areas, as no study has studied simultaneously these matters or done such an in-depth analysis. So, given the increasing importance of air pollution on AD and PD, the realities of European cities and their environmental and demographic problems, and the knowledge gaps mentioned above, this study has the value of covering the urge to develop more epidemiological works to understand the health effects of PM10 and PM2.5 on these diseases, namely, the acute situations that lead to the necessity of an emergency room visit. Furthermore, on a global scale, this study brings a thorough contribution to the short-term health effects of exposure to air pollutants on AD and PD, as there is only one study analyzing AD (Culqui et al. 2017), two evolving around PD (Goria et al. 2021; Lee et al. 2017), and one examining both diseases (Zanobetti et al. 2014).
This study aims to examine short-term exposure to PM10 and PM2.5 and its association with emergency hospital admissions due to AD and PD in the urban context, taking the city of Lisbon as a case study. Lisbon has the pinpoint conditions to make it a perfect case study for the European metropoles, as it has a dense urban fabric and very high traffic levels, combined with an aged population structure, considering that aged individuals are more likely to be diagnosed with any of these diseases. To develop this study, we followed a methodology based on multivariate Poisson regression models, a common methodology widely used and tested in studies of associations between exposure to air pollutants and population health.
Methods
Study area
The study was carried out in the city of Lisbon, the capital and largest city in Portugal with 506,892 inhabitants, from which 143,122 (26.6%) are over 65 years old (and where 14.4% of the total resident population is 75 years old), being the elderly Portuguese city (Instituto Nacional de Estatística 2022). The city is in the Lisbon Metropolitan Area (LMA), a metropolitan territory with the national highest level of emission of pollutants. The major contributor to air pollution is traffic (over 60% in LMA and a considerably higher share in Lisbon), followed, at a great distance, by other sectors: industry, energy production, and finally households’ combustion (CCDR LVT and FCT/UNL 2017; Torres et al. 2017).
This area was chosen due to its socioeconomic importance, metropolis characteristics, and exposure risk, but also for data availability. There is a consistent and complete health dataset in time and geographical frames and one of the best available air pollution and meteorological data.
Air pollution and meteorological data
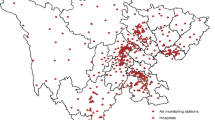
Concerning air pollutants, the study is focused on the concentration of PM10 and PM2.5 (μg/m3) for the period 2012 to 2015. These data were obtained through the Portuguese Environmental Agency Online Database on Air Quality (QualAr), an online tool that provides information on air quality indexes and pollutant concentration statistics at each monitoring station, through continuous measurements based on 1-h averages with data registration every 15 min. These data refer to six air pollution measuring stations within an urban environment located in the city of Lisbon (Fig. 1).
The PM10 and PM2.5 values used in the models were obtained through the construction of a daily average for each pollutant. Although having a generally good level of completion, there were some missing data (less than 5%). To achieve 100% data completion, and due to the somewhat residual percentage of missing data, the expectation-maximization algorithm was used (on the average daily values). The complete dataset made it possible to overcome the hypothetical biased estimates and the reduced power that would result from an analysis with the deletion of cases from missing exposures (Demissie et al. 2003).
In parallel to air pollution data, meteorological data such as temperature (°C), relative humidity (%), and wind speed (km/h) were obtained through the Iowa Environmental Mesonet (IEM), an online tool that collects environmental data from cooperating members with observation networks. Due to the importance of such variables in the assessment of health impacts (Areal et al. 2022; Su et al. 2021; Wei et al. 2019), these indicators were then used as control variables in the statistical analysis.
The selected station to retrieve data was “Lisboa-Portela,” and the information was transformed from hourly data to daily city averages for each of the meteorological variables used: temperature, wind speed, and relative humidity.
Emergency hospital admissions
Health data for the period 2012 to 2015 were obtained from the Central Administration of the Health System (ACSS) of the Portuguese Ministry of Health. Following the International Statistical Classification of Diseases, 9th Revision (ICD-9), and International Statistical Classification of Diseases, 10th Revision (ICD-10), individual daily admittance records for Alzheimer’s disease (ICD-9: 331.0; ICD-10: G30.9) and Parkinson’s disease (ICD-9: 332; ICD-10: G20 and G21) were considered for Lisbon resident population. From these records, only episodes associated with emergency department visits were retrieved, excluding the ones related to programmed admissions and other types of appointed visits. Furthermore, only the cases in which AD or PD was the main cause for emergency hospital admission were selected, excluding from the analysis the episodes that listed AD or PD as a secondary diagnosis. Hence, only the visits caused by AD or PD were used instead of all episodes that account for an individual that has these diseases but visits the emergency department due to other diagnostics.
Statistical analysis
Due to the characteristics of hospital admissions data (non-negative discrete numbers), emergency hospital admissions related to Alzheimer’s disease and Parkinson’s disease can be characterized as count data. The Kolmogorov–Smirnov test showed that the data was not normally distributed; hence, an ecological time series analysis with a multivariate Poisson regression model — a generalized linear model (GLM) — was used to examine the association between exposure to PM10 and PM2.5 and emergency hospital admissions due to Alzheimer’s disease and Parkinson’s disease. Furthermore, as there is virtually no overdispersion, which was attested by the ratio of the residual deviance and degrees of freedom (1.02) and further confirmed by the Cameron and Trivedi test for overdispersion, this statistical analysis method is preferable.
As it is known, exposure to air pollutants in 1 day does not necessarily lead to a hospital admission that same day, this can happen days later and may even be a result of the accumulated exposure in a given period. Therefore, we assessed the relationship on the same day of exposure to PM10 and PM2.5 and emergency hospital admissions (Lag 0), but also produced time-stratified analysis to contemplate other exposure timings, following the need for further investigation between shorter and longer lags evidenced by Lee et al. (2017): single lags, where data from air pollutants is from before the admission day, thus generating a delay of 1 day to 1 week (Lag 1, Lag 2, Lag 3, Lag 4, Lag 5, Lag 6, Lag 7; and cumulative lags or moving averages, where air pollutants data are the result of an average of the days prior to the admission, going from the case day and day before to 1-week exposure (Lag 0–1, Lag 0–2, Lag 0–3, Lag 0–4, Lag 0–5, and Lag 0–6).
The multivariate Poisson regression models can be specified as:
where log[E(Yt| X)] is the expected emergency hospital admissions on day t; β1t − l(PM10) and β2t − l(PM2.5) are the regression coefficients for each air pollutant at a given t-l (single-lag or cumulative lag); ns are natural smooth spline functions; TM corresponds to temperature; WS to wind speed; and RH to relative humidity. Air pollutants were linearly modeled as there was no sign of nonlinearity from the exploratory analysis or the model fitting. Also, Lee et al. (2017) thoroughly tested the linearity/nonlinearity and confirmed the linear relation. The degrees of freedom for meteorological variables were set to 3 as it showed the lowest Akaike’s information criterion (AIC) and went along with most studies on the impacts of air pollution on health (3 to 5 degrees of freedom). To account for trends and seasonality in the data, categorical variables for seasonality (SEASON — winter, spring, summer, and fall) and days of the week on the date of admission (DOW — Monday, Tuesday, Wednesday, Thursday, Friday, Saturday, and Sunday) were included. With these and the already mentioned inclusion of splines for meteorological variables, the autocorrelation and short-term variations were reduced, accommodating for non-linear patterns.
Also, stratified analyses by sex (male and female) and age (early and late elderly, less than 75 years old and 75 years or older) were performed to identify if these conditions are a potential factor for a higher/lower risk of emergency hospital admission. The statistical significance for the differences between estimates of potential effect modifier was tested through the calculation of 95% confidence intervals (Payton et al. 2003):
where \({\hat{Q}}_1\) and \({\hat{Q}}_2\) are the estimates for each sex/age group \({\hat{SE}}_1^2\) and \({\hat{SE}}_2^2\) and are the standard errors.
The null hypothesis was rejected if the interval did not contain zero (Payton et al. 2003; Schenker and Gentleman 2001).
Like other studies (C. Chen et al. 2018; Nuntavarn et al. 2008; Wan Mahiyuddin et al. 2013), we examined the sensitivity of key findings concerning the specification of degrees of freedom in the meteorological variables (df = 3, 4, and 5), observing model stability. Also, the obtained results were examined with the development of zero-inflated Poisson (ZIP) models, checking the model suitability.
Results are expressed in percentage change in emergency hospital admissions due to AD or PD with its 95% confidence interval (CI) for a 10 μg/m3 increase in PM2.5 and PM10.
The analysis was developed with the following statistical software and their packages: IBM SPSS Statistics 27 and R (4.0.5) Statistical Computing Environment.
Results
Descriptive analysis
In the 1461 days that compose the studied period, from January 1, 2012, to December 31, 2015, 2117 emergency hospital admissions for AD (64.7% female and 35.3% male) and 3508 for PD (51.4% female and 48.6% male) were registered. Considering the European air quality guidelines suggested in Directive 2008/50/EC, only 89 daily averages of PM10 concentration occurrences (6.1% of total observations) exceeded the limit of 50 μg/m3. According to the limits defined by the same directive, and to great surprise due to Lisbon’s urban characteristics, there are no exceedances of PM2.5 for the period under analysis. Even when the at the time WHO threshold is considered (daily PM2.5 averages must not exceed the 25 μg/m3 value), only 70 days exceeded the guidelines (4.8%). However, when considering the most recent WHO guidelines (where the annual average concentrations of PM2.5 should not exceed 5 μg/m3, and 24-h average exposures should not exceed 15 μg/m3 more than 3 to 4 days per year), the situation changes, as the annual averages from the 2012 to 2015 period fluctuate from 10.89 to 12.99 μg/m3 and the days in which the 24-h average exceeds the 15 μg/m3 limit vary from 73 to 114 per year. In general, emergency hospital admissions tend to follow the pollutants behavior. Figure 2 illustrates the seasonality of air pollutants and emergency hospital admissions, as was already expected, there are higher levels of PM emissions in the cold months (winter in the northern hemisphere).
Furthermore, we found that air pollutants and environmental variables were not correlated, or at most poorly correlated (r. 0–0.32). Table 1 shows the descriptive statistics for emergency hospital admissions due to AD and PD and the environmental control variables for the period 2012–2015.
Short-term exposure association between PM and AD and PD emergency hospital admissions
The Poisson regression models incorporated PM10 and PM2.5; however, only the latter consistently presented an increase in the risk of emergency hospital admission, even though there are no legal exceedances in this air pollutant for the studied period.
We found a great number of statistically significant associations between air pollutants and emergency hospital admissions. Figure 3 shows the p-values for all AD and PD models. The diseases have different behaviors regarding the time-stratified analysis. While AD shows less statistically significant associations with admissions, being less sensitive to single lag effects, only Lag 1 shows statistical significance, and in cumulative lags, the significance decreases as the period grows; PD not only does it show more models with statistically significant associations, but it has a different behavior regarding time-stratified analysis, with the models with greater statistical significance appearing from further single lags (Lag 5) and longer cumulative lags (Lag 0–5 and Lag 0–6).
The biggest percentage increases in emergency hospital admissions for a 10 μg/m3 increase in PM2.5 are felt on cumulative lags. Table 2 shows the percent increase in emergency hospital admissions for a 10 μg/m3 increase in PM2.5 across all time-stratified structures. The difference between AD and PD is in the period in which these values occur, while in AD, it is in shorter cumulative lags (decreasing from Lag 0–1), in PD, it is in longer periods (increasing from Lag 0–1).
We found that at a Lag 0 observation, AD shows one of the highest percent increases for admissions with a 3.75% (CI 95% 1.91–5.63) for a 10 μg/m3 increase in PM2.5, only being surpassed by Lag 0–1 stratification 4.09% (CI 95% 2.00–6.21). As for PD, the Lag 0 model has one of the lowest percentage increases for admissions 1.77% (CI 95% 0.35–3.21), while the largest percent increase is shown by Lag 0–6 with 4.38% (CI 95% 2.34–6.45) for a 10 μg/m3 increase in PM2.5.
This suggests that for short-term exposure to PM2.5 and its impacts on emergency hospital admissions, AD and PD are differently affected. AD is less impacted by longer lagged observations, being its effects most felt right after exposure at Lag 0 and Lag 1 (being Lag 5 the exception, nevertheless showing a much reduced percentage increase than the shorter lags), and at shorter cumulative lags. As for PD, it seems to have a delayed impact, with stronger increases in Lag 2 to Lag 5, while also being more susceptible to longer cumulative lags.
Stratified analysis for demographic characteristics
Figure 4 shows the percentage increase in AD and PD for the demographic characteristics in Lag 5, Lag 0–3, and Lag 0–4. Although men consistently show stronger effects on emergency hospital admissions for the increment in PM2.5, there was no statistically significant difference. As for age, it was found that it presented significant evidence of being a modifier. This happened for individuals over 75 years of age and just for PD, with an increase in emergency hospital admissions for a 10 μg/m3 increase in PM2.5 of 2.49% (95% CI 0.71–4.30) (p-value = 0.006) in Lag 5, 3.69% (1.22–6.20) (p-value = 0.003) in Lag 0–3, and 3.23% (95% CI 0.67–5.84) (p-value = 0.013) in Lag 0–4. Figure 4 only reports the lags that have a p<0.005 to enhance its visualization.
Sensitivity and validity of the results
We found that the models are quite robust. Neither the choice of degrees of freedom nor the choice of a statistical method produced any major impact on the outcomes, as the results are practically identical in the ZIP models (results not shown). The difference in the percentage increase in hospital admissions is between 0.0 and 0.2%, depending on the model. This not only reflects the robustness of the analysis but also the very small to null dispersion in the data.
Discussion
In this ecological time series study, we assessed and confirmed the existence of a statistically significant association between short-term exposure to air pollutants and emergency hospital admissions for AD and PD. Even though not establishing these associations as an etiological root of either of the diseases, we assessed that an increment in emissions is associated with the increase in emergency hospital admissions, being not a cause but a trigger for an episode requiring a visit to the emergency room. The examined pollutants were PM10 and PM2.5, in the case of the former one, it was found no effect on admissions, notwithstanding having slightly more exceedances throughout the analyzed period. As for PM2.5, it was found an effect on emergency hospital admissions, in a context of not existing (legal) or very low daily exceedances (WHO threshold) — although having a greater number of exceeding values per day and in annual averages within the scope of the updated WHO guidelines — stressing the need to understand air pollutants effects on health even when emission levels are below the limit. This is especially interesting as there is a scarcity of studies involving short-term exposure to PM2.5 and its effects on hospital admissions (or mortality) due to both diseases. From our results, it seems that PD is more sensitive to longer cumulative lags, whereas AD shows more immediate impacts from increased exposure. Also, we did not find any statistically significant effect modifier from demographic characteristics for AD; however, for PD, age is a modifier.
Most of the previous studies have undertaken a methodology based on long-term exposure to pollutants emissions; therefore, if a comparison between the present work and such analyses should be done, the conclusions must be viewed with some caution. These studies compiled evidence on continued exposure to air pollutants and the relation with these mental disorders and, in general, a connection was found. In a study for the city of Ontario, it was found a 4% increase in incident PD (95% CI 1.01–1.08) for an IQR increment in PM2.5 emissions (Shin et al. 2018). In other work, this time for New York, it was observed an association of PM2.5 with PD first hospitalization with an RR of 1.09 (95% CI 1.04–1.14) for an increase in emissions from 8.1 to 10.4 μg/m3, while also gathering evidence of an effective association with AD [34]. In a thorough and comprehensive review, it was found an increased risk of AD 3.26 (95% 0.84–12.74) and PD 1.34 (95% CI 1.04–1.73) for augmentations in PM2.5 exposure (Nunez et al. 2021). Moreover, these results are in line with the findings of the present study in terms of association and, in a more scattered manner (as they are already among them), in the matter of quantifiable percentage/risk associated.
Worldwide, only 5 studies, including this one, address the association between short-term exposure to particulate matter and admissions for AD or PD, being from Culqui et al. (2017) (AD), Goria et al. (2021) (PD), Lee et al. (2017) (PD), and Zanobetti et al. (2014) (AD and PD).
The first study to emerge was from (Zanobetti et al. 2014), this was a national case-crossover analysis in the USA that included 121 different-sized communities. They found a 3.23% (95% CI: 1.08, 5.43) increase in emergency hospital admissions for PD and 0.20% (95% CI −1.26–1.69) for AD in a 10 μg/m3 increase in 2-day average concentrations, although the values for AD were not statistically significant. Nevertheless, these values are distinct from the 1.90% and 4.09%, respectively, found in this study. These differences may be related to topographic, demographic, and climate characteristics, but they may also derive from the analysis itself, Zanobetti et al. (2014) observed 121 communities, very different from each other and, consequently, with large variations between them, which combined with cases of missing data results in greater p-values and accordingly estimates. Also, they found that PD had only an association with smaller lags, while we found associations for greater lags and observed stronger effects on them. Additionally, in contrast to the present study, they found significant evidence of effect modification for age in AD, with a 3.48% (95% CI: 0.83, 6.19) increase in subjects aged between 65 and 75 years, while we found it for PD and individuals aged 75 years or over. Regarding time-stratifications, shorter lags produce stronger effects, being in line with our findings for AD but not for PD.
The previous study was followed by Lee et al. (2017) with the city of Seoul as the background and PD as the objective. Despite focusing only on one city, like our study, the dissimilarities are quite large. Seoul is a bigger and denser city, being demographically different from Lisbon (younger population), topographically different (Seoul is a basin surrounded by mountains while Lisbon is a coastal hilly city), but also presenting much higher pollution values (more than twice Lisbon’s PM2.5 annual averages). As for the results, they found more statistically significant associations between air pollution and PD than Zanobetti et al. (2014) and much stronger effects; for instance, at the same lag mentioned above, they found an increase of 6.18%, even though without statistical significance. Their analysis, in general, had results far higher than those found so far in any other study, with its maximum odds ratio of 1.61 (95% CI 1.14–2.29) for the largest cumulative lag (8 days), even when the accessory diagnosis is accounted, the odds ratio remains above the values of 1.1. Also, they show possible higher impacts on women than on men, which goes against the findings from Zanobetti et al. (2014) and our results; however, none of these analyses are statistically significant. Furthermore, as it happens in our study, Lee et al. (2017) findings tend to show the strongest effects for the longer-lagged pollutants concentrations, differing from Zanobetti et al. (2014) and reiterating the further need to evaluate the associations between short-term exposure and PD at other territorial realities.
Also in 2017, Culqui et al. (2017) studied AD and short-term exposure in Madrid, a city different from Lisbon or Seoul, as it is a continental city, but closer to Lisbon regarding density, population age structure, and pollution levels. In their analysis, Culqui et al. (2017) focused on the effect of heat waves and PM2.5 on PD hospitalizations, having a lateral focus on PM. They found an association between PM2.5 and PD, at a 2-day lag for a relative risk of 1.38 (95% CI 1.15–1.65) for an increment in inter-quartile range (IQR) 20 μg/m3 on PM2.5 concentration. If we attend to their increase in PM2.5 and calculate a hypothetical relative risk as theirs, our results are somewhat similar, especially at Lag 0. This is in accordance with Zanobetti et al. (2014), stronger results are obtained in more immediate lags; nonetheless, we emphasize the need to further develop this analysis in future studies as many results, in all studies, lacked statistical significance.
The most recent work, from Goria et al. (2021), addresses short-term exposure to air pollutants and the risk of hospital admission due to PD in 18 French areas. As was the case of Zanobetti et al. (2014), they have studied many territories (albeit considerably less) and such dissimilarities among them increment the variations. Also, they could not distinguish between planned and unplanned visits, nor give consistent results of sample stratification, like sex or age. The differences from our case study, Lisbon, are quite large. They found the lowest relative risk 1.010 (95% CI 1.003–1.017) of PD hospital admissions per 10 μg/m3 increase in PM2.5 across all known studies. Furthermore, the study design is focused on seasonality, finding a larger effect on PD in spring and summer, just as Lee et al. (2017) found.
Our study found a clear distinction of PM2.5 impacts on emergency hospital admissions among AD and PD. This is a very significant finding as the effects of lagged analysis, as already stressed, needed more assessments, and our results proved to be very consistent. We observed that AD shows greater percent increases at shorter lags and PD shows greater increases at more lengthy lags, which is in line with the findings of Lee et al. (2017) for PD and Zanobetti et al. (2014) for AD, albeit our findings are noticeably sturdier. These differentiated effects could be related to the processes that generate the oxidative stress and the promotion of the formation of protein aggregates, and the specific brain region that is associated with each disease, such as the hippocampus and cerebral cortex for AD and substantia nigra for PD (Chin-Chan et al. 2015). The time comprised between exposure to air pollutants and the point at which the individuals seek the hospital emergency room may differ. In fact, in PD, such situation seems to mainly come from accumulated exposure. However, further studies, not only epidemiological, are needed to assess the reality behind these differentiated effects on both diseases.
Based on our findings and the ones from previous studies, it is plausible to support the existence of a relationship between emergency hospital admissions due to AD and PD and an increase in air pollutant concentrations. However, it still seems to be very precarious to postulate what this relation is and how it works, not only because findings show a tendency to be more similar on the regional spectrum than on the global one (for instance the similarities among Iberian cities) but much also because there are still very few studies.
As for limitations, the biggest drawback of this study and others similar in Portugal is data availability. It is not common to have health data this precise and scrutinized in Portugal; however, some aspects inherent to a study such as this are still difficult to overcome, mainly the inexistence of any kind of socioeconomic information regarding the individuals’ admissions, making it impossible to study these conditions as effect modifiers or even using them as control variables. In addition, this is an ecological study, it has the advantage of high numbers across the whole population, but it also has the disadvantage of possible misdiagnosis, that is, cases that were AD or PD events yet not diagnosed as such (for several reasons). Environmental data are only consistently available from a certain point in time, making it impossible to evaluate the situation further back in time and make use of the complete database of air pollutants emissions and hospital admissions. Another pertinent aspect to mention is the reality of air pollution measuring stations, they exist, but they are well distributed and have reliable data in Lisbon city (not even in its suburbs), making it impossible for the study to expand to other cities or communities due to lack of data, either due to the lack of measuring stations or data inconsistency (especially in PM10 and PM2.5).
Conclusions
We found that, unlike PM10, there is an association between short-term PM2.5 exposure and a percentage increase in emergency hospital admission due to AD and PD. Although not having found any statistically significant effect modifier for sex, age emerged as a consistent modifier for PD, being the risk greater for men and, in terms of disease, for AD. Also, we reiterate (Lee et al. 2017) findings in lagged observations for PD, as it showed to be less sensitive to immediate lags and more to longer cumulative lags. For AD, the contrary was found. These associations were assessed in a context of no legal exceedances in daily PM2.5 emissions, drawing attention to the need to rethink the cities relationship with air pollution and its sources, but mainly with traffic, as it is the main source of air pollution for cities throughout the world.
From a public health standpoint, these findings are particularly important for aging cities with high levels of traffic and air pollution, as the European cities are, calling for attention to AD and PD and short-term PM2.5 exposure while focusing on high-risk subgroups. This is further reiterated in a post-pandemic context, where combating pollution needs to be a pressing matter in the planning for healthier and more livable cities.
Data availability
The data that support the findings of this study are available from the Central Administration of the Health System (ACSS) of the Portuguese Ministry of Health, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of Central Administration of the Health System (ACSS) of the Portuguese Ministry of Health.
References
Areal AT, Zhao Q, Wigmann C, Schneider A, Schikowski T (2022) The effect of air pollution when modified by temperature on respiratory health outcomes: a systematic review and meta-analysis. Sci Total Environ 811:152336. https://doi.org/10.1016/j.scitotenv.2021.152336
Attademo L, Bernardini F (2017) Air pollution and urbanicity: common risk factors for dementia and schizophrenia? Lancet Planet Health 1(3):e90–e91. https://doi.org/10.1016/S2542-5196(17)30042-6
Aturinde A, Farnaghi M, Pilesjö P, Sundquist K, Mansourian A (2021) Spatial analysis of ambient air pollution and cardiovascular disease (CVD) hospitalization across Sweden. GeoHealth 5(5):e2020GH000323. https://doi.org/10.1029/2020GH000323
Babadjouni RM, Hodis DM, Radwanski R, Durazo R, Patel A, Liu Q, Mack WJ (2017) Clinical effects of air pollution on the central nervous system; a review. J Clin Neurosci 43:16–24. https://doi.org/10.1016/j.jocn.2017.04.028
Bandyopadhyay A (2016) Neurological disorders from ambient (urban) air pollution emphasizing UFPM and PM2.5. Curr Pollut Rep 2(3):203–211. https://doi.org/10.1007/s40726-016-0039-z
Block ML, Calderón-Garcidueñas L (2009) Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci 32(9):506–516. Elsevier Current Trends. https://doi.org/10.1016/j.tins.2009.05.009
Boda E, Rigamonti AE, Bollati V (2020) Understanding the effects of air pollution on neurogenesis and gliogenesis in the growing and adult brain. Current Opinion in Pharmacology 50:61–66. https://doi.org/10.1016/j.coph.2019.12.003
Brito J, Bernardo A, Zagalo C, Gonçalves LL (2021) Quantitative analysis of air pollution and mortality in Portugal: current trends and links following proposed biological pathways. Sci Total Environ 755:142473. https://doi.org/10.1016/j.scitotenv.2020.142473
Cacciottolo M, Morgan TE, Saffari AA, Shirmohammadi F, Forman HJ, Sioutas C, Finch CE (2020) Traffic-related air pollutants (TRAP-PM) promote neuronal amyloidogenesis through oxidative damage to lipid rafts. Free Radic Biol Med 147:242–251. https://doi.org/10.1016/j.freeradbiomed.2019.12.023
Calderón-Garcidueñas L, Azzarelli B, Acuna H, Garcia R, Gambling TM, Osnaya N, Monroy S, Tizapantzi MDR, Carson JL, Villarreal-Calderon A, Rewcastle B (2002) Air pollution and brain damage. Toxicol Pathol 30(3):373–389. https://doi.org/10.1080/01926230252929954
Calderón-Garcidueñas L, Reed W, Maronpot RR, Henríquez-Roldán C, Delgado-Chavez R, Calderón-Garcidueñas A, Dragustinovis I, Franco-Lira M, Aragón-Flores M, Solt AC, Altenburg M, Torres-Jardón R, Swenberg JA (2004) Brain inflammation and Alzheimer’s-like pathology in individuals exposed to severe air pollution. Toxicol Pathol 32(6):650–658. https://doi.org/10.1080/01926230490520232
Calderón-Garcidueñas L, Reynoso-Robles R, González-Maciel A (2019) Combustion and friction-derived nanoparticles and industrial-sourced nanoparticles: the culprit of Alzheimer and Parkinson’s diseases. Environ Res 176:108574. https://doi.org/10.1016/j.envres.2019.108574
Calderón-Garcidueñas L, Torres-Jardón R, Kulesza RJ, Mansour Y, González-González LO, Gónzalez-Maciel A, Reynoso-Robles R, Mukherjee PS (2020) Alzheimer disease starts in childhood in polluted Metropolitan Mexico City. A major health crisis in progress. Environ Res 183:109–137. https://doi.org/10.1016/j.envres.2020.109137
Camacho I, Camacho J, Camacho R, Góis A, Nóbrega V (2020) Influence of outdoor air pollution on cardiovascular diseases in Madeira (Portugal). Water, Air, Soil Pollut 231(3):1–15. https://doi.org/10.1007/s11270-020-4430-4
CCDR LVT, & FCT/UNL (2017) Inventário de emissões atmosféricas da região de Lisboa e Vale do Tejo | 2011 - 2014. http://www.ccdr-lvt.pt/files/f6a975f1d2a0ba5974fded0bbac285b30f0fb53f.pdf
Chen C, Zhu P, Lan L, Zhou L, Liu R, Sun Q, Ban J, Wang W, Xu D, Li T (2018) Short-term exposures to PM2.5 and cause-specific mortality of cardiovascular health in China. Environ Res 161:188–194. https://doi.org/10.1016/j.envres.2017.10.046
Chen H, Kwong JC, Copes R, Tu K, Villeneuve PJ, van Donkelaar A, Hystad P, Martin RV, Murray BJ, Jessiman B, Wilton AS, Kopp A, Burnett RT (2017) Living near major roads and the incidence of dementia, Parkinson’s disease, and multiple sclerosis: a population-based cohort study. Lancet 389(10070):718–726. https://doi.org/10.1016/S0140-6736(16)32399-6
Chin-Chan M, Navarro-Yepes J, Quintanilla-Vega B (2015) Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front Cell Neurosci 9:124. https://doi.org/10.3389/fncel.2015.00124
Culqui DR, Linares C, Ortiz C, Carmona R, Díaz J (2017) Association between environmental factors and emergency hospital admissions due to Alzheimer’s disease in Madrid. Sci Total Environ 592:451–457. https://doi.org/10.1016/j.scitotenv.2017.03.089
Demissie S, LaValley MP, Horton NJ, Glynn RJ, Cupples LA (2003) Bias due to missing exposure data using complete-case analysis in the proportional hazards regression model. Stat Med 22(4):545–557. https://doi.org/10.1002/sim.1340
Donaldson K, Tran L, Jimenez LA, Duffin R, Newby DE, Mills N, MacNee W, Stone V (2005) Combustion-derived nanoparticles: a review of their toxicology following inhalation exposure. Particle Fibre Toxicol 2(1):1–14. BioMed Central. https://doi.org/10.1186/1743-8977-2-10
Ferreira JJ, Gonçalves N, Valadas A, Januário C, Silva MR, Nogueira L, Vieira JLM, Lima AB (2017) Prevalence of Parkinson’s disease: a population-based study in Portugal. Eur J Neurol 24(5):748–750. https://doi.org/10.1111/ene.13273
Franco P, Gordo C, Marques da Costa E, Lopes A (2020) Air pollution and emergency hospital admissions—evidences from Lisbon Metropolitan Area, Portugal. Appl Sci 10(22):7997. https://doi.org/10.3390/app10227997
Franco P, Marques da Costa E (2021) Atividade física no quotidiano familiar das periferias.: Uma visão a partir de Rio de Mouro–Sintra. Finisterra 56(116):183–203. https://doi.org/10.18055/Finis20067
Fu P, Guo X, Cheung FMH, Yung KKL (2019) The association between PM 2.5 exposure and neurological disorders: a systematic review and meta-analysis. Sci Total Environ 655:1240–1248. https://doi.org/10.1016/j.scitotenv.2018.11.218
Ghio AJ, Smith CB, Madden MC (2012) Diesel exhaust particles and airway inflammation. Curr Opin Pulm Med 18(2) https://journals.lww.com/co-pulmonarymedicine/Fulltext/2012/03000/Diesel_exhaust_particles_and_airway_inflammation.10.aspx
Goria S, Pascal M, Corso M, Le Tertre A (2021) Short-term exposure to air pollutants increases the risk of hospital admissions in patients with Parkinson’s disease – a multicentric study on 18 French areas. Atmos Environ 264:118668. https://doi.org/10.1016/j.atmosenv.2021.118668
Gunn LD, Mavoa S, Boulangé C, Hooper P, Kavanagh A, Giles-Corti B (2017) Designing healthy communities: creating evidence on metrics for built environment features associated with walkable neighbourhood activity centres. Int J Behav Nutr Phys Act 14(1):164. https://doi.org/10.1186/s12966-017-0621-9
Hahad O, Lelieveld J, Birklein F, Lieb K, Daiber A, Münzel T (2020) Ambient air pollution increases the risk of cerebrovascular and neuropsychiatric disorders through induction of inflammation and oxidative stress. International Journal of Molecular Sciences 21(12):4306. https://doi.org/10.3390/ijms21124306
He MZ, Do V, Liu S, Kinney PL, Fiore AM, Jin X, DeFelice N, Bi J, Liu Y, Insaf TZ, Kioumourtzoglou M-A (2021) Short-term PM2.5 and cardiovascular admissions in NY State: assessing sensitivity to exposure model choice. Environ Health 20(1):93. https://doi.org/10.1186/s12940-021-00782-3
Hu CY, Fang Y, Li FL, Dong B, Hua XG, Jiang W, Zhang H, Lyu Y, Zhang XJ (2019) Association between ambient air pollution and Parkinson’s disease: systematic review and meta-analysis. Environ Res 168:448–459. https://doi.org/10.1016/j.envres.2018.10.008
Instituto Nacional de Estatística. (2022) Statistical database. https://www.ine.pt/xportal/xmain?xpid=INE&xpgid=ine_base_dados&contexto=bd&selTab=tab2
Jin L, Zhou T, Fang S, Zhou X, Han B, Bai Y (2022) The short-term effects of air pollutants on pneumonia hospital admissions in Lanzhou, China, 2014–2019: evidence of ecological time-series study. Air Qual Atmos Health. https://doi.org/10.1007/s11869-022-01244-6
Karbakhsh M, Mansourian M, Taheri M, Rabiei K, Hosseini SM, Rahimi M, Sadeghian B, Chan S, Sarrafzadegan N, Brauer M (2022) Outdoor fine and coarse particles and hospital admissions for cardiovascular diseases: a large-scale case-crossover study. Air Qual Atmos Health 15(9):1679–1693. https://doi.org/10.1007/s11869-022-01212-0
Kasdagli MI, Katsouyanni K, Dimakopoulou K, Samoli E (2019) Air pollution and Parkinson’s disease: a systematic review and meta-analysis up to 2018. Int J Hyg Environ Health 222(3):402–409. https://doi.org/10.1016/j.ijheh.2018.12.006
Khorrami Z, Ye T, Sadatmoosavi A, Mirzaee M, Fadakar Davarani MM, Khanjani N (2020) The indicators and methods used for measuring urban liveability: a scoping review. Rev Environ Health. https://doi.org/10.1515/reveh-2020-0097
Kilian J, Kitazawa M (2018) The emerging risk of exposure to air pollution on cognitive decline and Alzheimer’s disease – evidence from epidemiological and animal studies. Biomed J 41(3):141–162. Elsevier B.V. https://doi.org/10.1016/j.bj.2018.06.001
Kioumourtzoglou M-A, Schwartz JD, Weisskopf MG, Melly SJ, Wang Y, Dominici F, Zanobetti A (2016) Long-term PM 2.5 exposure and neurological hospital admissions in the Northeastern United States. Environ Health Perspect 124(1):23–29. https://doi.org/10.1289/ehp.1408973
Lee H, Myung W, Kim DK, Kim SE, Kim CT, Kim H (2017) Short-term air pollution exposure aggravates Parkinson’s disease in a population-based cohort. Sci Rep 7(1):1–10. https://doi.org/10.1038/srep44741
Liu R, Young MT, Chen JC, Kaufman JD, Chen H (2016) Ambient air pollution exposures and risk of Parkinson disease. Environ Health Perspect 124(11):1759–1765. https://doi.org/10.1289/EHP135
Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, Costafreda SG, Dias A, Fox N, Gitlin LN, Howard R, Kales HC, Kivimäki M, Larson EB, Ogunniyi A, Mukadam N (2020) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet 396(10248):413–446. Lancet Publishing Group. https://doi.org/10.1016/S0140-6736(20)30367-6
Louro, A., Franco, P., & Marques da Costa, E. (2021a) Determinants of physical activity practices in metropolitan context: the case of Lisbon Metropolitan Area, Portugal. Sustainability 13(18). https://doi.org/10.3390/su131810104
Louro, A., Marques da Costa, N., & Marques da Costa, E. (2019) Sustainable urban mobility policies as a path to healthy cities—the case study of LMA, Portugal. In Sustainability (Vol. 11, Issue 10). https://doi.org/10.3390/su11102929
Louro A, Marques da Costa N, Marques da Costa E (2021b) From livable communities to livable metropolis: challenges for urban mobility in Lisbon Metropolitan Area (Portugal). Int J Environ Res Public Health 18(7). https://doi.org/10.3390/ijerph18073525
Mallhi TH, Butt MH, Ahmad A, Misbah S, Atique S, Khan YH (2021) In: Akash MSH, Rehman K (eds) Air pollutants and neurological disorders: from exposure to preventive interventions BT - environmental contaminants and neurological disorders. Springer International Publishing, pp 31–47. https://doi.org/10.1007/978-3-030-66376-6_2
Martins A, Scotto M, Deus R, Monteiro A, Gouveia S (2021) Association between respiratory hospital admissions and air quality in Portugal: a count time series approach. PLOS One 16(7):e0253455. https://doi.org/10.1371/journal.pone.0253455
Mir RH, Sawhney G, Pottoo FH, Mohi-ud-din R, Madishetti S, Jachak SM, Ahmed Z, Masoodi MH (2020) Role of environmental pollutants in Alzheimer’s disease: a review. Environ Sci Pollut Res 27(36):44724–44742. https://doi.org/10.1007/s11356-020-09964-x
Moulton PV, Yang W (2012) Air pollution, oxidative stress, and Alzheimer’s disease. J Environ Publ Health 2012. Hindawi Publishing Corporation. https://doi.org/10.1155/2012/472751
Nunez Y, Boehme AK, Weisskopf MG, Re DB, Navas-Acien A, van Donkelaar A, Martin RV, Kioumourtzoglou M-A (2021) Fine particle exposure and clinical aggravation in neurodegenerative diseases in New York State. Environ Health Perspect 129(2):027003. https://doi.org/10.1289/EHP7425
Nuntavarn V-V, Nitaya V, Bart O (2008) The Public Health and Air Pollution in Asia (PAPA) project: estimating the mortality effects of particulate matter in Bangkok. Thailand. Environmental Health Perspectives 116(9):1179–1182. https://doi.org/10.1289/ehp.10849
Payton ME, Greenstone MH, Schenker N (2003) Overlapping confidence intervals or standard error intervals: what do they mean in terms of statistical significance? J Insect Sci 3(1). https://doi.org/10.1093/jis/3.1.34
Peng W, Li H, Peng L, Wang Y, Wang W (2022) Effects of particulate matter on hospital admissions for respiratory diseases: an ecological study based on 12.5 years of time series data in Shanghai. Environ Health 21(1):12. https://doi.org/10.1186/s12940-021-00828-6
Peters R, Ee N, Peters J, Booth A, Mudway I, Anstey KJ (2019) Air pollution and dementia: a systematic review. J Alzheimer’s Dis 70(s1):S145–S163). IOS Press. https://doi.org/10.3233/JAD-180631
Power MC, Adar SD, Yanosky JD, Weuve J (2016) Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: a systematic review of epidemiologic research. NeuroToxicology 56:235–253. https://doi.org/10.1016/j.neuro.2016.06.004
Pringsheim T, Jette N, Frolkis A, Steeves TDL (2014) The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Move Disord 29(13):1583–1590. https://doi.org/10.1002/mds.25945
Rodrigues M, Natário I, do Rosário de Oliveira Martins M (2021) Estimate the effects of environmental determining factors on childhood asthma hospital admissions in Lisbon, Portugal: a time series modelling study. Theor Appl Climatol 143(1):809–821. https://doi.org/10.1007/s00704-020-03415-w
Rodríguez D, Cobo-Cuenca A, Quiles R (2022) Effects of air pollution on daily hospital admissions for cardiovascular diseases in Castilla-La Mancha, Spain: a region with moderate air quality. Air Quality, Atmosphere & Health 15:591–604. https://doi.org/10.1007/s11869-021-01144-1
Santana I, Farinha F, Freitas S, Rodrigues V, Carvalho Á (2015) The epidemiology of dementia and Alzheimer disease in Portugal: estimations of prevalence and treatment-costs. Acta Méd Port 28(2). https://doi.org/10.20344/amp.6025
Schenker N, Gentleman JF (2001) On judging the significance of differences by examining the overlap between confidence intervals. The American Statistician 55(3):182–186. https://doi.org/10.1198/000313001317097960
Schikowski T, Altuğ H (2020) The role of air pollution in cognitive impairment and decline. Neurochem Int (Vol. 136, p. 104708). Elsevier Ltd. https://doi.org/10.1016/j.neuint.2020.104708
Shehab MA, Pope FD (2019) Effects of short-term exposure to particulate matter air pollution on cognitive performance. Sci Rep 9(1):8237. https://doi.org/10.1038/s41598-019-44561-0
Shin S, Burnett RT, Kwong JC, Hystad P, van Donkelaar A, Brook JR, Copes R, Tu K, Goldberg MS, Villeneuve PJ, Martin RV, Murray BJ, Wilton AS, Kopp A, Chen H (2018) Effects of ambient air pollution on incident Parkinson’s disease in Ontario, 2001 to 2013: a population-based cohort study. Int J Epidemiol 47(6):2038–2048. https://doi.org/10.1093/ije/dyy172
Shou Y, Huang Y, Zhu X, Liu C, Hu Y, Wang H (2019) A review of the possible associations between ambient PM2.5 exposures and the development of Alzheimer’s disease. Ecotoxicology and Environmental Safety 174:344–352. https://doi.org/10.1016/j.ecoenv.2019.02.086
Sicard P, Agathokleous E, De Marco A, Paoletti E, Calatayud V (2021) Urban population exposure to air pollution in Europe over the last decades. Environ Sci Eur 33(1):28. https://doi.org/10.1186/s12302-020-00450-2
Su X, Song H, Cheng Y, Yao X, Li Y (2021) The mortality burden of nervous system diseases attributed to ambient temperature: a multi-city study in China. Science of The Total Environment 800:149548. https://doi.org/10.1016/j.scitotenv.2021.149548
Tarín-Carrasco P, Im U, Geels C, Palacios-Peña L, Jiménez-Guerrero P (2021) Contribution of fine particulate matter to present and future premature mortality over Europe: a non-linear response. Environ Int 153:106517. https://doi.org/10.1016/j.envint.2021.106517
Torres P, Costa S, Ferreira J, Silveira C, Miranda AI, Teixeira JP, Pereira MDC, Mendes A (2017) Poluição atmosférica: breve revisão da situação em Portugal e os impactos na saúde pública. Bol Epidemiol Observações 6(19):20–25
Vujcic M, Tomicevic-Dubljevic J, Zivojinovic I, Toskovic O (2019) Connection between urban green areas and visitors’ physical and mental well-being. Urban For Urban Green 40:299–307. https://doi.org/10.1016/j.ufug.2018.01.028
Wan Mahiyuddin WR, Sahani M, Aripin R, Latif MT, Thach T-Q, Wong C-M (2013) Short-term effects of daily air pollution on mortality. Atmos Environ 65:69–79. https://doi.org/10.1016/j.atmosenv.2012.10.019
Wang C, Wolters PJ, Calfee CS, Liu S, Balmes JR, Zhao Z, Koyama T, Ware LB (2020) Long-term ozone exposure is positively associated with telomere length in critically ill patients. Environ Int 141:105780. https://doi.org/10.1016/j.envint.2020.105780
Wei Y, Wang Y, Lin C-K, Yin K, Yang J, Shi L, Li L, Zanobetti A, Schwartz JD (2019) Associations between seasonal temperature and dementia-associated hospitalizations in New England. Environ Int 126:228–233. https://doi.org/10.1016/j.envint.2018.12.054
Woodward N, Finch CE, Morgan TE (2015) Traffic-related air pollution and brain development. AIMS Environ Sci 2(2):353–373. https://doi.org/10.3934/environsci.2015.2.353
Yee J, Cho YA, Yoo HJ, Yun H, Gwak HS (2021) Short-term exposure to air pollution and hospital admission for pneumonia: a systematic review and meta-analysis. Environ Health 20(1):6. https://doi.org/10.1186/s12940-020-00687-7
Younan D, Petkus AJ, Widaman KF, Wang X, Casanova R, Espeland MA, Gatz M, Henderson VW, Manson JE, Rapp SR, Sachs BC, Serre ML, Gaussoin SA, Barnard R, Saldana S, Vizuete W, Beavers DP, Salinas JA, Chui HC, Chen JC (2020) Particulate matter and episodic memory decline mediated by early neuroanatomic biomarkers of Alzheimer’s disease. Brain 143(1):289–302. https://doi.org/10.1093/brain/awz348
Yu H-R, Lin C-HR, Tsai J-H, Hsieh Y-T, Tsai T-A, Tsai C-K, Lee Y-C, Liu T-Y, Tsai C-M, Chen C-C, Chang C-H, Hsu T-Y, Niu C-K (2020) A multifactorial evaluation of the effects of air pollution and meteorological factors on asthma exacerbation. Int J Environ Res Public Health 17(11):4010. https://doi.org/10.3390/ijerph17114010
Yu Z, Wei F, Zhang X, Wu M, Lin H, Shui L, Jin M, Wang J, Tang M, Chen K (2021) Air pollution, surrounding green, road proximity and Parkinson’s disease: a prospective cohort study. Environ Res 197:111170. https://doi.org/10.1016/j.envres.2021.111170
Yuchi W, Sbihi H, Davies H, Tamburic L, Brauer M (2020) Road proximity, air pollution, noise, green space and neurologic disease incidence: a population-based cohort study. Environ Health 19(1):8. https://doi.org/10.1186/s12940-020-0565-4
Zanobetti A, Dominici F, Wang Y, Schwartz JD (2014) A national case-crossover analysis of the short-term effect of PM2.5 on hospitalizations and mortality in subjects with diabetes and neurological disorders. Environ Health: Glob Access Sci Source 13(1):1–11. https://doi.org/10.1186/1476-069X-13-38
Zhang Y, Ding Z, Xiang Q, Wang W, Huang L, Mao F (2020) Short-term effects of ambient PM1 and PM2.5 air pollution on hospital admission for respiratory diseases: case-crossover evidence from Shenzhen, China. Int J Hyg Environ Health 224:113418. https://doi.org/10.1016/j.ijheh.2019.11.001
Zhang Y, Gu Z (2013) Air quality by urban design. Nat Geosci 6(7):506. https://doi.org/10.1038/ngeo1869
Funding
Open access funding provided by FCT|FCCN (b-on). Pedro Franco has a PhD Grant (SFRH/BD/138999/2018) supported by Fundação para a Ciência e Tecnologia (FCT, Portugal). This work was developed in the frame of the Metropolitan Plan for Adaptation to Climate Change – Lisbon Metropolitan Area (PMAAC-AML) and Grampcity (Project PTDC/GES-TRA/32121/2017).
Author information
Authors and Affiliations
Contributions
Pedro Franco: conceptualization; data curation; investigation; methodology; formal analysis; writing — original draft; and visualization. Cristina Gordo: data curation; investigation; validation; and visualization. Eduarda Marques da Costa: conceptualization; supervision; and writing — original draft. António Lopes: conceptualization; supervision; and writing — original draft.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Franco, P., Gordo, C., Marques da Costa, E. et al. Short-term exposure to particulate matter and effects on emergency hospital admissions for Alzheimer’s disease and Parkinson’s disease: an ecological study from an aged European metropolis. Air Qual Atmos Health 16, 1619–1631 (2023). https://doi.org/10.1007/s11869-023-01359-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-023-01359-4