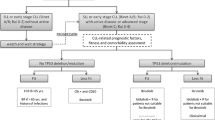
Opinion statement
Chronic lymphocytic leukemia (CLL) is the most common leukemia in adults. Most individuals diagnosed with CLL will not need treatment immediately but over time the clonal B cells infiltrate the bone marrow, lymph nodes, liver, and spleen, causing anemia, thrombocytopenia, systemic symptoms, and increased risk for infections. When clonal B cells begin adversely affecting other organs, treatment is warranted. Therapy for CLL has undergone a paradigm shift away from chemotherapy-based regimens to targeted therapy with small-molecule inhibitors. B-cell receptor (BCR) signaling plays a key role in CLL. BCR signaling occurs via many factors including Bruton’s tyrosine kinase (BTK), phosphatidylinositol 3-kinase (PI3K), phosphatidylinositol-4,5-bisphosphonate phosphodiesterase gamma-2 (PLCγ2), and CD19. CLL cells also express high levels of B-cell lymphoma or leukemia 2 (BCL2). Drugs that interfere with these pathways, such as ibrutinib, venetoclax, and idelalisib, have improved clinical outcomes. For any CLL patient that meets criteria for treatment, after evaluating for prognostic cytogenetic abnormalities, oral BTK inhibitors or venetoclax in combination with anti-CD20 therapy are considered first-line therapy. It is important to note that these novel therapies are particularly preferred for patients with TP53 mutations or deletion of the small arm of chromosome 17 (del(17p)), as those patients usually are chemotherapy refractory or display short remissions to chemotherapy. Nevertheless, patients without high-risk features such as TP53 abnormalities also benefit from novel agents. Following relapse, depending on the primary oral agent used, BTK inhibitors, venetoclax in combination with anti-CD20 antibodies, or PI3K inhibitors are preferred.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
National Comprehensive Cancer Network. Chronic lymphocytic leukemia/small lymphocytic lymphoma (version 3.2021). https://www.nccn.org/professionals/physician_gls/pdf/cll.pdf. Accessed 06/04/2021.
Soumerai JD, Ni A, Darif M, Londhe A, Xing G, Mun Y, et al. Prognostic risk score for patients with relapsed or refractory chronic lymphocytic leukaemia treated with targeted therapies or chemoimmunotherapy: a retrospective, pooled cohort study with external validations. Lancet Haematol. 6:e366–74. https://doi.org/10.1016/S2352-3026(19)30085-7.
Sharman JP, Egyed M, Jurczak W, Skarbnik A, Pagel JM, Flinn IW, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzumab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395:1278–91. https://doi.org/10.1016/S0140-6736(20)30262-2.
Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghiaet P, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015; 373.25:2425–2437.
•• Burger JA, Barr PM, Robak TOC, Ghia P, Tedeschi A, et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2020;34:787–98. https://doi.org/10.1038/s41375-019-0602-x Randomized study showing high-risk CLL patients (TP53, IGHV mutation, etc.) had improved benefit with ibrutinib when compared to chlorambucil.
Moreno C, Greil R, Demirkan F, Tedeschi A, Anz B, Larratt L, et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukemia (iLLUMINATE): a multicentre, randomized, open-label, phase 3 trial. Lancet Oncol. 2019;20:43–56. https://doi.org/10.1016/S1470-2045(18)30788-5.
Ujjani C, Mato A, Hill BT, Allan JN, Lansigan F, Jacobs R, et al. The impact of age on survival in CLL patients receiving ibrutinib as initial therapy. Blood Lymphat Cancer. 2020;10:1–5. https://doi.org/10.2147/BLCTT.S262592.
Ghia P, Pluta A, Wach M, Lysak D, Kozak T, Simkovic M, et al. ASCEND: phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2020;38:2849–61. https://doi.org/10.1200/JCO.19.03355.
Xu W, Yang S, Zhou K, Pan L, Li A, Zhou J, et al. Treatment of relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma with the BTK inhibitor zanubrutinib: phase 2, single-arm, multicenter study. J Hematol Oncol. 2020;13(48). https://doi.org/10.1186/s13045-020-00884-4.
Tam CS, Robak T, Ghia P, Kahl BS, Walker P, Janowski W, et al. Zanubrutinib monotherapy for patients with treatment naïve chronic lymphocytic leukemia and 17p deletion. Haematologica. 2020 Oct 13; Online ahead of print. https://doi.org/10.3324/haematol.2020.259432.
Brown JR, Robak T, Ghia P, Kahl BS, Walker P, Janowki W, et al. Efficacy and safety of zanubrutinib in patients with treatment naïve (TN) chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) with del(17p): follow-up results from arm C of the SEQUOIA (BGB-3111-304) trial. Poster presentation #1306. 62nd American Society of Hematology (ASH) Annual Meeting and Exposition; Dec 5, 2020; Virtual.
Tam CS, Trotman J, Opat S, Burger JA, Cull G, Gottlieb D. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019 Sep 12;134(11):851–9. https://doi.org/10.1182/blood.2019001160.
Tam CS, Opat S, Simpson D, Cull G, Munoz J, Phillips TJ. Zanubrutinib for the treatment of relapsed or refractory mantle cell lymphoma. Blood Adv. 2021;5(12):2577–85. https://doi.org/10.1182/bloodadvances.2020004074.
Trotman J, Opat S, Gottlieb D, Simpson D, Marlton P, Cull G, Munoz J, Tedeschi A, Roberts AW, Seymour JF, Atwal SK, Yu Y, Novotny W, Holmgren E, Tan Z, Hilger JD, Huang J, Tam CS. Zanubrutinib for the treatment of patients with Waldenström macroglobulinemia: 3 years of follow-up. Blood. 2020 Oct 29;136(18):2027–37. https://doi.org/10.1182/blood.2020006449.
•• Al-Sawaf O, Zhang C, Tandon M, Sinha A, Fink AM, Robrecht S, et al. Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (CLL14): follow-up results from a multicentre, open-label, randomized, phase 3 trial. Lancet Oncol. 2020;21:1188–200. https://doi.org/10.1016/S1470-2045(20)30443-5 Randomized study showed that venetoclax plus obinutuzumab had significantly improved outcomes when compared to chlorambucil plus obinutuzumab, including the high-risk CLL patients (TP53, IGHV mutation, etc.).
Tam CS, Siddiqi T, Allan JN, Kipps TJ, Kuss BJ, Opat S, et al. Ibrutinib (Ibr) plus venetoclax (Ven) for first-line treatment of chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL): results from the MRD cohort of the phase 2 CAPTIVATE study. Blood. 2019;134:35. https://doi.org/10.1182/blood-2019-121424.
Wierda WG, Tam CS, Allan JN, Siddiqi T, Kipps TJ, Opat S, et al. Ibrutinib (Ibr) plus venetoclax (Ven) for first-line treatment of chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL): 1-year results from the MRD cohort of the phase 2 CAPTIVATE study. Oral abstract #123. 62nd ASH Annual Meeting and Exposition; Dec 5, 2020; Virtual.
Mato AR. Approaches for relapsed CLL after chemotherapy-free frontline regimens. ASH 2020 annual meeting. https://www.hematology.org/meetings/annual-meeting/programs/education-program
Harrup RA, Owen C, D'Rozario J, Robak T, Kater AP, Montillo M, et al. Efficacy of subsequent novel targeted therapies, including repeated venetoclax-rituximab (VenR), in patients (Pts) with relapsed/refractory chronic lymphocytic leukemia (R/R CLL) previously treated with fixed-duration Venr in the Murano study. Blood. 2020;136:44–5. https://doi.org/10.1182/blood-2020-137415.
Mato AR, Hill BT, Lamanna N, Barr PM, Ujjani CS, Brander DM, et al. Optimal sequencing of ibrutinib, idelalisib, and venetoclax in chronic lymphocytic leukemia: results from a multicenter study of 683 patients. Ann Oncol. 2017 May 1;28(5):1050–6. https://doi.org/10.1093/annonc/mdx031.
Mato AR, Roeker LE, Jacobs R, Hill BT, Lamanna N, Brander D, et al. Assessment of the efficacy of therapies following venetoclax discontinuation in CLL reveals BTK inhibition as an effective strategy. Clin Cancer Res. 2020 Jul 15;26(14):3589–96. https://doi.org/10.1158/1078-0432.CCR-19-3815.
Lin VS, Lew TE, Handunnetti SM, Blombery P, Nguyen T, Westerman DA, et al. BTK inhibitor therapy is effective in patients with CLL resistant to venetoclax. Blood. 2020;135:2266–70. https://doi.org/10.1182/blood.2020004782.
Munir T, Brown JR, O'Brien S, Barrientos JC, Barr PM, Reddy NM, et al. Final analysis from RESONATE: up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am Hematol. 2019;94:1353–63. https://doi.org/10.1002/ajh.25638.
Byrd JC, Wierda WG, Schuh A, Devereux S, Chaves JM, Brown JR, et al. Acalabrutinib monotherapy in patients with relapsed/refractory chronic lymphocytic leukemia: updated phase 2 results. Blood. 2020;135:1204–13. https://doi.org/10.1182/blood.2018884940.
Awan FT, Schuh A, Brown JR, Furman RR, Pagel JM, Hillmen P, Stephens DM, Woyach J, Bibikova E, Charuworn P, Frigault MM, Hamdy A, Izumi R, Linghu B, Patel P, Wang MH, Byrd JC. Acalabrutinib monotherapy in patients with chronic lymphocytic leukemia who are intolerant to ibrutinib. Blood Adv. 2019 May 14;3(9):1553–62. https://doi.org/10.1182/bloodadvances.
•• Byrd JC, Hillmen P, Ghia P, Kater AP, Chanan-Khan AAA, Furman RR. First results of a head-to-head trial of acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia. J Clin Oncol. 2021;39(15_suppl):7500–0. https://doi.org/10.1200/JCO.2021.39.15_suppl.7500 Study comparing acalabrutinib versus ibrutinib showed similar efficacy but less side effects with acalabrutinib.
Hillman P, Eichhorst B, Brown JR, Lamanna N, O’Brien S, Tam CS, et al. First interim analysis of ALPINE study: results of a phase 3 randomized study of zanubrutinib vs ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. Presidential symposium. Abstract #LB1900. EHA2021; June 11, 2020.
Stilgenbauer S, Eichhorst B, Schetelig J, Coutre S, Seymour JF, Munir T, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol. 2016;17:768–78. https://doi.org/10.1016/S1470-2045(16)30019-5.
Seymour JF, Kipps TJ, Eichhorst B, Hillmen P, D’Rozario J, Assouline S, et al. Venetoclax–rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378:1107–20. https://doi.org/10.1056/NEJMoa1713976.
Kater AP, Wu JQ, Kipps T, Eichhorst B, Hillmen P, D'Rozario J, et al. Venetoclax plus rituximab in relapsed chronic lymphocytic leukemia: 4-year results and evaluation of impact of genomic complexity and gene mutations from the MURANO Phase III Study. J Clin Oncol. 2020;38:4042–54. https://doi.org/10.1200/JCO.20.00948.
Kater AP, Kipps TJ, Eichhorst B, Hillmen P, D’Rozario J, Owen C, et al. Five-year analysis of Murano study demonstrates enduring undetectable minimal residual disease (uMRD) in a subset of relapsed/refractory chronic lymphocytic leukemia (R/R CLL) patients following fixed-duration venetoclax-rituximab (VenR) therapy. Oral Abstract. Abstract # 125. 62nd ASH annual meeting and exposition; December 5, 2020.
Coutre S, Choi M, Furman RR, Eradat H, Heffner L, Jones JA, et al. Venetoclax for patients with chronic lymphocytic leukemia who progressed during or after idelalisib therapy. Blood. 2018;131:1704–11. https://doi.org/10.1182/blood-2017-06-788133.
Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007. https://doi.org/10.1056/NEJMoa1315226.
Flinn IW, Hillmen P, Montillo M, Nagy Z, Illés Á, Etienne G, et al. The phase 3 DUO trial: duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood. 2018;13:2446–55. https://doi.org/10.1182/blood-2018-05-850461.
Davids MS, Kuss BJ, Hillmen P, Montillo M, Moreno C, Essell J, et al. Efficacy and safety of duvelisib following disease progression on ofatumumab in patients with relapsed/refractory CLL or SLL in the DUO crossover extension study. Clin Cancer Res. 2020;26:2096–103. https://doi.org/10.1158/1078-0432.CCR-19-3061.
Munoz J, Follows GA, Nastoupil LJ. Copanlisib for the treatment of malignant lymphoma: clinical experience and future perspectives. Target Oncol. 2021 May;16(3):295–308. https://doi.org/10.1007/s11523-021-00802-9.
Dreyling M, Santoro A, Mollica L, Leppä S, Follows G, Lenz G, Kim WS, Nagler A, Dimou M, Demeter J, Özcan M, Kosinova M, Bouabdallah K, Morschhauser F, Stevens DA, Trevarthen D, Munoz J, Rodrigues L, Hiemeyer F, et al. Long-term safety and efficacy of the PI3K inhibitor copanlisib in patients with relapsed or refractory indolent lymphoma: 2-year follow-up of the CHRONOS-1 study. Am J Hematol. 2020 Apr;95(4):362–71. https://doi.org/10.1002/ajh.25711.
Zinzani PL, Santoro A, Mollica L, Leppä S, Follows GA, Lenz G, Kim WS, Nagler A, Panayiotidis P, Demeter J, Morschhauser F, Munoz J, Miriyala A, Benson A, Garcia-Vargas J, Childs BH, Dreyling M. Copanlisib, a PI3K inhibitor, demonstrates a favorable long-term safety profile in a pooled analysis of patients with hematologic malignancies. Blood. 2019;134(Supplement_1):4009. https://doi.org/10.1182/blood-2019-131779.
ClincalTrials.gov[Internet]. Bethesda (MD): National Library of Medicine (US). July 2021- . Identifier NCT04685915, Copanlisib plus ibrutinib in R/R CLL; cited 2021 Sept 01; Available from: https://clinicaltrials.gov/ct2/show/NCT04685915
Roeker LE, Dreger P, Brown JR, Lahoud OB, Eyre TA, Brander DM, et al. Allogeneic stem cell transplantation for chronic lymphocytic leukemia in the era of novel agents. Blood Adv. 2020;4:3977–89. https://doi.org/10.1182/bloodadvances.2020001956.
Kim HT, Shaughnessy CJ, Rai SC, Reynolds C, Ho VT, Cutler C, et al. Allogeneic hematopoietic cell transplantation after prior targeted therapy for high-risk chronic lymphocytic leukemia. Blood Adv. 2020;4:4113–23. https://doi.org/10.1182/bloodadvances.2020002184.
Morschhauser F, Dyer MJS, Walter HS, Danilov AV, Ysebaert L, Hodson DJ, et al. Phase 1b study of tirabrutinib in combination with idelalisib or entospletinib in previously treated B-cell lymphoma. Leukemia. 2020. https://doi.org/10.1038/s41375-020-01108-x.
Danilov AV, Herbaux C, Walter HS, Hillmen P, Rule SA, Kio EA, et al. Phase Ib study of tirabrutinib in combination with idelalisib or entospletinib in previously treated chronic lymphocytic leukemia. Clin Cancer Res. 2020;26:2810–8. https://doi.org/10.1158/1078-0432.CCR-19-3504.
Mato AR, Ghosh N, Schuster SJ, Lamanna N, Pagel JM, Flinn IW, et al. Phase 2 study of the safety and efficacy of umbralisib in patients with CLL who are intolerant to BTK or PI3Kδ inhibitor therapy. Blood. 2020 Dec 1:blood.2020007376. https://doi.org/10.1182/blood.2020007376.
Gribben JG, Jurczak W, Jacobs RW, Grosicki S, Giannopoulou K, Wrobel T, et al. Umbralisib plus ublituximab (U2) is superior to obinutuzumab plus chlorambucil (O+Chl) in patients with treatment naïve (TN) and relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL): results from the phase 3 Unity-CLL study. Blood. 2020;136:37–9. https://doi.org/10.1182/blood-2020-134783.
Pollyea DA, Coutre S, Gore L, Adler N, Harris P, Phelps MA. A dose escalation study of ibrutinib with lenalidomide for relapsed and refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. Blood. 2014;124(21):1987. https://doi.org/10.1182/blood.V124.21.1987.1987.
Ujjani C, Wang H, Skarbnik A, Trivedi N, Ramzi P, Khan N. A phase 1 study of lenalidomide and ibrutinib in combination with rituximab in relapsed and refractory CLL. Blood Adv. 2018 Apr 10;2(7):762–8. https://doi.org/10.1182/bloodadvances.2017015263.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–44. https://doi.org/10.1056/NEJMoa1707447.
Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382:1331–42. https://doi.org/10.1056/NEJMoa1914347.
Munoz J, Jaglowski S, McKinney MS, Isufi I, Stiff PJ, Sachs J. A phase 1 study of ACTR087 in combination with rituximab, in subjects with relapsed or refractory CD20-positive B-cell lymphoma. Blood. 2019;134(Supplement_1):244. https://doi.org/10.1182/blood-2019-123810.
Geyer MB, Rivière I, Sénéchal B, Wang X, Wang Y, Purdon TJ, et al. Safety and tolerability of conditioning chemotherapy followed by CD19-targeted CAR T cells for relapsed/refractory CLL. JCI Insight. 2019;5:e122627. https://doi.org/10.1172/jci.insight.122627.
Turtle CJ, Hay KA, Hanafi LA, Li D, Cherian S, Chen X, et al. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J Clin Oncol. 2017;35:3010–20. https://doi.org/10.1200/JCO.2017.72.8519.
Siddiqi T, Dorritie KA, Soumerai JD, Stephans DM, Dubovsky JA, Gillenwater HH, et al. TRANSCEND CLL 004: minimal residual disease (MRD) negative responses after lisocabtagene maraleucel (Liso-Cel; JCAR017), a CD19-directed CAR T cell product, in patients (pts) with relapsed/refractory chronic lymphocytic leukemia or small lymphocytic lymph. J Clin Oncol. 2019;37:7501. https://doi.org/10.1200/JCO.2019.37.15_suppl.7501.
Wierda WG, Dorritie KA, Munoz J, Stephens DM, Solomon SR, Gillenwater HH. Transcend CLL 004: phase 1 cohort of lisocabtagene maraleucel (liso-cel) in combination with ibrutinib for patients with relapsed/refractory (R/R) chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). ASH 2020. Abstract.
Gauthier J, Hirayama AV, Purushe J, Hay KA, Lymp J, Li DH, et al. Feasibility and efficacy of CD19-targeted CAR T cells with concurrent ibrutinib for CLL after ibrutinib failure. Blood. 2020;135:1650–60. https://doi.org/10.1182/blood.2019002936.
Cappell KM, Sherry RM, Yang JC, Goff SL, Vanasse DA, McIntyre L, et al. Long-term follow-up of anti-CD19 chimeric antigen receptor T-cell therapy. J Clin Oncol. 2020;38:3805–15. https://doi.org/10.1200/JCO.20.01467.
Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med. 2020;382:545–53. https://doi.org/10.1056/NEJMoa1910607.
Horwitz SM, Feldman TA, Hess BT, Khodadoust MS, Kim YA, Munoz J. The novel SYK/JAK inhibitor cerdulatinib demonstrates good tolerability and clinical response in a phase 2a study in relapsed/refractory peripheral T-cell lymphoma and cutaneous T-cell lymphoma. Blood. 2018;132(Supplement 1):1001. https://doi.org/10.1182/blood-2018-99-119944.
Smith SD, Munoz J, Stevens D, Smith SM, Feldman TA, Ye JC. Rapid and durable responses with the SYK/JAK inhibitor cerdulatinib in a phase 2 study in relapsed/refractory follicular lymphoma-alone or in combination with rituximab. Blood. 2019;134(Supplement_1):3981. https://doi.org/10.1182/blood-2019-124231.
Hamlin PA, Cheson BD, Farber CM, Feldman T, Fenske TS, Hess BT. The dual SYK/JAK inhibitor cerdulatinib demonstrates rapid tumor responses in a phase 2 study in patients with relapsed/refractory B- and T-cell non-Hodgkin lymphoma (NHL). J Clin Oncol. 2018;36(15_suppl):7511–1. https://doi.org/10.1200/JCO.2018.36.15_suppl.7511.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Lymphoma
Rights and permissions
About this article
Cite this article
Wiedmeier-Nutor, J., Leis, J. Chronic Lymphocytic Leukemia: Chemotherapy Free and Other Novel Therapies Including CAR T. Curr. Treat. Options in Oncol. 23, 904–919 (2022). https://doi.org/10.1007/s11864-022-00953-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-022-00953-5