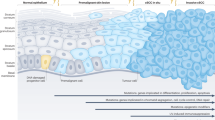
Opinion statement
The capacity of cells to modify their phenotypes from epithelial to mesenchymal (epithelial-to-mesenchymal transition or EMT) and vice versa provides them with a dynamic plasticity essential for human life, from embryogenesis to wound healing. Current knowledge about carcinogenetic mechanisms leaves little doubts on the pivotal participation of these interchangeable processes in cancer development, and their influence has been quite clearly established in the progression of cutaneous squamous cell carcinoma. A complex and ordered interplay of signals induces the shift between both phenotypes, providing cells with the most suitable state at every moment to face the next step in tumor invasion and dissemination. Some stimulatory triggers have opposite effects according to the biological context and in many cases exert collateral functions. This scenario makes finding an ideal therapeutic target difficult but provides the opportunity to intervene simultaneously at many different levels with small actions such as targeting the tumor environment. In any case, advances in knowledge of the EMT mechanisms and their influence on carcinogenesis and drug resistance will greatly influence the therapeutic strategies for many human tumors, including cutaneous squamous cell carcinoma.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bertrand K. Survival of exfoliated epithelial cells: a delicate balance between anoikis and apoptosis. J Biomed Biotechnol. 2011;2011:534139.
Iradyan M, Iradyan N, Hulin P, et al. Targeting degradation of EGFR through the allosteric site leads to cancer cell detachment-promoted death. Cancers (Basel). 2019. https://doi.org/10.3390/cancers11081094.
Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel). 1995;154:8–20.
Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–96.
Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8.
Eggenhofer E, Luk F, Dahlke MH, Hoogduijn MJ. The life and fate of esenchymal stem cells. Front Immunol. 2014. https://doi.org/10.3389/fimmu.2014.00148.
Song L, Tuan RS. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J. 2004;18:980–2.
Block CJ, Dyson G, Campeanu IJ, Watza D, Ratnam M, Wu G. A stroma-corrected ZEB1 transcriptional signature is inversely associated with antitumor immune activity in breast cancer. Sci Rep. 2019;9:17807.
Barriere G, Fici P, Gallerani G, Fabbri F, Rigaud M. Epithelial mesenchymal transition: a double-edged sword. Clin Transl Med. 2015;4:1–6. https://doi.org/10.1186/s40169-015-0055-4.
Acloque H, Thiery JP, Nieto MA. The physiology and pathology of the EMT. Meeting on the epithelial–mesenchymal transition. EMBO Rep. 2008;9:322–6.
Stone RC, Pastar I, Ojeh N, Chen V, Liu S, Garzon KI, et al. Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res. 2016;365:495–506.
Wolska-Gawron K, Bartosińska J, Krasowska D. MicroRNA in localized scleroderma: a review of literature. Arch Dermatol Res. 2019:1–8. https://doi.org/10.1007/s00403-019-01991-0.
Li X, Zhang Z, Zhang Y, Cao Y, Wei H, Wu Z. Upregulation of lactate-inducible snail protein suppresses oncogene-mediated senescence through p16INK4a inactivation. J Exp Clin Cancer Res. 2018;37:39.
Polette M, Mestdagt M, Bindels S, Nawrocki-Raby B, Hunziker W, Foidart J-M, et al. Beta-catenin and ZO-1: shuttle molecules involved in tumor invasion-associated epithelial-mesenchymal transition processes. Cells Tissues Organs. 2007;185:61–5.
Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–72.
Murtas D, Maxia C, Diana A, Pilloni L, Corda C, Minerba L, et al. Role of epithelial-mesenchymal transition involved molecules in the progression of cutaneous melanoma. Histochem Cell Biol. 2017;148:639–49.
Li Z-R, Jiang Y, Hu J-Z, Chen Y, Liu Q-Z. SOX2 knockdown inhibits the migration and invasion of basal cell carcinoma cells by targeting the SRPK1-mediated PI3K/AKT signaling pathway. Oncol Lett. 2019;17:1617–25.
Xu W, Yang Z, Lu N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell Adhes Migr. 2015;9:317–24.
Nieszporek A, Skrzypek K, Adamek G, Majka M. Molecular mechanisms of epithelial to mesenchymal transition in tumor metastasis. Acta Biochim Pol. 2019;66:509–20.
Hao Y, Baker D, ten Dijke P. TGF-β-mediated epithelial-mesenchymal transition and cancer metastasis. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20112767.
Feng J, Byrne NM, Al Jamal W, Coulter JA. Exploiting current understanding of hypoxia mediated tumour progression for nanotherapeutic development. Cancers (Basel). 2019. https://doi.org/10.3390/cancers11121989.
Yeo CD, Kang N, Choi SY, Kim BN, Park CK, Kim JW, et al. The role of hypoxia on the acquisition of epithelial-mesenchymal transition and cancer stemness: a possible link to epigenetic regulation. Korean J Intern Med. 2017;32:589–99.
Chaudhary S, Islam Z, Mishra V, Rawat S, Ashraf GM, Kolatkar PR. Sox2: a regulatory factor in tumorigenesis and metastasis. Curr Protein Pept Sci. 2019;20:495–504.
Expósito-Villén A, E Aránega A, Franco D. Functional role of non-coding RNAs during epithelial-to-mesenchymal transition. Noncoding RNA. 2018. https://doi.org/10.3390/ncrna4020014.
Renganathan A, Felley-Bosco E. Long noncoding RNAs in cancer and therapeutic potential. Adv Exp Med Biol. 2017;1008:199–222.
Innao V, Allegra A, Pulvirenti N, Allegra AG, Musolino C. Therapeutic potential of antagomiRs in haematological and oncological neoplasms. Eur J Cancer Care (Engl). 2020:e13208.
Gugnoni M, Ciarrocchi A. Long noncoding RNA and epithelial mesenchymal transition in cancer. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20081924.
Jia D, Li X, Bocci F, Tripathi S, Deng Y, Jolly MK, et al. Quantifying cancer epithelial-mesenchymal plasticity and its association with stemness and immune response. J Clin Med. 2019. https://doi.org/10.3390/jcm8050725.
Li C, Balazsi G. A landscape view on the interplay between EMT and cancer metastasis. NPJ Syst Biol Appl. 2018;4:34.
Celià-Terrassa T, Jolly MK. Cancer stem cells and epithelial-to-mesenchymal transition in cancer metastasis. Cold Spring Harb Perspect Med. 2019. https://doi.org/10.1101/cshperspect.a036905.
Wang H, Unternaehrer JJ. Epithelial-mesenchymal transition and cancer stem cells: at the crossroads of differentiation and dedifferentiation. Dev Dyn. 2019;248:10–20.
Takaishi M, Tarutani M, Takeda J, Sano S. Mesenchymal to epithelial transition induced by reprogramming factors attenuates the malignancy of cancer cells. PLoS One. 2016;11:e0156904.
Wang B, Tan Z, Guan F. Tumor-derived exosomes mediate the instability of cadherins and promote tumor progression. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20153652.
Fiori ME, Di Franco S, Villanova L, Bianca P, Stassi G, De Maria R. Cancer-associated fibroblasts as abettors of tumor progression at the crossroads of EMT and therapy resistance. Mol Cancer. 2019;18:70.
Suarez-Carmona M, Lesage J, Cataldo D, Gilles C. EMT and inflammation: inseparable actors of cancer progression. Mol Oncol. 2017;11:805–23.
Ferrari N, Ranftl R, Chicherova I, et al. Dickkopf-3 links HSF1 and YAP/TAZ signalling to control aggressive behaviours in cancer-associated fibroblasts. Nat Commun. 2019;10:130.
•• Debaugnies M, Sánchez-Danés A, Rorive S, Raphaël M, Liagre M, Parent M-A, et al. YAP and TAZ are essential for basal and squamous cell carcinoma initiation. EMBO Rep. 2018. https://doi.org/10.15252/embr.201845809 Demonstrates the importance of YAP and TAZ on cancer initiation and specifically the role of YAP on epithelial-to-mesenchymal transition, suggesting that these can be targetable drugs.
Chan JW, Yeh I, El-Sayed IH, Algazi AP, Glastonbury CM, Ha PK, et al. Ultraviolet light-related DNA damage mutation signature distinguishes cutaneous from mucosal or other origin for head and neck squamous cell carcinoma of unknown primary site. Head Neck. 2019;41:E82–5.
Pickering CR, Zhou JH, Lee JJ, Drummond JA, Peng SA, Saade RE, et al. Mutational landscape of aggressive cutaneous squamous cell carcinoma. Clin Cancer Res. 2014;20:6582–92.
Dotto GP, Rustgi AK. Squamous cell cancers: a unified perspective on biology and genetics. Cancer Cell. 2016;29:622–37.
• García-Sancha N, Corchado-Cobos R, Pérez-Losada J, Cañueto J. MicroRNA dysregulation in cutaneous squamous cell carcinoma. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20092181 Excellent review on the role of microRNA in the development and spread of Cutaneous Squamous Cell Carcinoma including informative diagrams by experts in this topic.
Yu X, Li Z. The role of miRNAs in cutaneous squamous cell carcinoma. J Cell Mol Med. 2016;20:3–9.
Wang A, Landén NX, Meisgen F, Lohcharoenkal W, Ståhle M, Sonkoly E, et al. MicroRNA-31 is overexpressed in cutaneous squamous cell carcinoma and regulates cell motility and colony formation ability of tumor cells. PLoS One. 2014;9:e103206.
Toll A, Salgado R, Espinet B, Díaz-Lagares A, Hernández-Ruiz E, Andrades E, et al. MiR-204 silencing in intraepithelial to invasive cutaneous squamous cell carcinoma progression. Mol Cancer. 2016;15:53.
Mizrahi A, Barzilai A, Gur-Wahnon D, et al. Alterations of microRNAs throughout the malignant evolution of cutaneous squamous cell carcinoma: the role of miR-497 in epithelial to mesenchymal transition of keratinocytes. Oncogene. 2018;37:218–30.
Moses MA, George AL, Sakakibara N, Mahmood K, Ponnamperuma RM, King KE, et al. Molecular mechanisms of p63-mediated squamous cancer pathogenesis. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20143590.
•• Robinson DJ, Patel A, Purdie KJ, et al. Epigenetic regulation of iASPP-p63 feedback loop in cutaneous squamous cell carcinoma. J Invest Dermatol. 2019;139:1658–1671.e8 Highlights the importance of epithelial-to-mesenchymal transition among the pleiotropic influences of p63 and its epigenetic regulation.
Kadota K, Nitadori J, Rekhtman N, Jones DR, Adusumilli PS, Travis WD. Reevaluation and reclassification of resected lung carcinomas originally diagnosed as squamous cell carcinoma using immunohistochemical analysis. Am J Surg Pathol. 2015;39:1170–80.
Smirnov A, Anemona L, Novelli F, Piro CM, Annicchiarico-Petruzzelli M, Melino G, et al. p63 is a promising marker in the diagnosis of unusual skin cancer. Int J Mol Sci. 2019;20:5781.
Matin RN, Chikh A, Chong SLP, et al. p63 is an alternative p53 repressor in melanoma that confers chemoresistance and a poor prognosis. J Exp Med. 2013;210:581–603.
Nirenberg A, Steinman H, Dixon J, Dixon A. Merkel cell carcinoma update: the case for two tumours. J Eur Acad Dermatol Venereol. 2019. https://doi.org/10.1111/jdv.16158.
Hu W-M, Jin J-T, Wu C-Y, Lu J-B, Zhang L-H, Zeng J, et al. Expression of P63 and its correlation with prognosis in diffuse large B-cell lymphoma: a single center experience. Diagn Pathol. 2019;14:128.
Romano R-A Novel genetic models to study the role of DNp63 in squamous cell carcinoma.
Chikh A, Matin RNH, Senatore V, et al. iASPP/p63 autoregulatory feedback loop is required for the homeostasis of stratified epithelia. EMBO J. 2011;30:4261–73.
Oh J-E, Kim RH, Shin K-H, Park N-H, Kang MK. DeltaNp63α protein triggers epithelial-mesenchymal transition and confers stem cell properties in normal human keratinocytes. J Biol Chem. 2011;286:38757–67.
Zhao W, Wang H, Han X, et al. ΔNp63α attenuates tumor aggressiveness by suppressing miR-205/ZEB1-mediated epithelial-mesenchymal transition in cervical squamous cell carcinoma. Tumour Biol. 2016;37:10621–32.
Siljamäki E, Rappu P, Riihilä P, Nissinen L, Kähäri V-M, Heino J. H-Ras activation and fibroblast-induced TGF-β signaling promote laminin-332 accumulation and invasion in cutaneous squamous cell carcinoma. Matrix Biol. 2019. https://doi.org/10.1016/j.matbio.2019.09.001.
Condorelli AG, Dellambra E, Logli E, Zambruno G, Castiglia D. Epidermolysis bullosa-associated squamous cell carcinoma: from pathogenesis to therapeutic perspectives. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20225707.
Yuen WY, Jonkman MF. Risk of squamous cell carcinoma in junctional epidermolysis bullosa, non-Herlitz type: report of 7 cases and a review of the literature. J Am Acad Dermatol. 2011;65:780–9.
Shiga K, Hara M, Nagasaki T, Sato T, Takahashi H, Takeyama H. Cancer-associated fibroblasts: their characteristics and their roles in tumor growth. Cancers (Basel). 2015;7:2443–58.
Martins VL, Vyas JJ, Chen M, Purdie K, Mein CA, South AP, et al. Increased invasive behaviour in cutaneous squamous cell carcinoma with loss of basement-membrane type VII collagen. J Cell Sci. 2009;122:1788–99.
Li XM, Kim SJ, Hong D-K, Jung KE, Choi CW, Seo Y-J, et al. KLF4 suppresses the tumor activity of cutaneous squamous cell carcinoma (SCC) cells via the regulation of SMAD signaling and SOX2 expression. Biochem Biophys Res Commun. 2019;516:1110–5.
Sun F, Hu K. Krüppel-like factor 4 inhibits the transforming growth factor-β1-promoted epithelial-to-mesenchymal transition via downregulating plasminogen activator inhibitor-1 in lung epithelial cells. Dis Markers. 2015;2015:473742.
Zhang Q-L, Li XM, Lian D-D, Zhu MJ, Yim S-H, Lee J-H, et al. Tumor suppressive function of NQO1 in cutaneous squamous cell carcinoma (SCC) cells. Biomed Res Int. 2019;2019:2076579.
Zhou S, da Silva SD, Siegel PM, Philip A. CD109 acts as a gatekeeper of the epithelial trait by suppressing epithelial to mesenchymal transition in squamous cell carcinoma cells in vitro. Sci Rep. 2019;9:16317.
Vincent-Mistiaen Z, Elbediwy A, Vanyai H, Cotton J, Stamp G, Nye E, et al. YAP drives cutaneous squamous cell carcinoma formation and progression. Elife. 2018. https://doi.org/10.7554/eLife.33304.
Cantariño N, Fernández-Figueras MT, Valero V, Musulén E, Malinverni R, Granada I, et al. A cellular model reflecting the phenotypic heterogeneity of mutant HRAS driven squamous cell carcinoma. Int J Cancer. 2016;139:1106–16.
Febres-Aldana CA, Alvarez Moreno JC, Rivera M, Kaplan S, Paramo J, Poppiti R. Understanding the histogenesis of a HRAS-PIK3R1 co-driven metastatic metaplastic breast carcinoma associated with squamous metaplasia of lactiferous ducts. Pathol Int. 2019. https://doi.org/10.1111/pin.12887.
Bordignon P, Bottoni G, Xu X, et al. Dualism of FGF and TGF-β signaling in heterogeneous cancer-associated fibroblast activation with ETV1 as a critical determinant. Cell Rep. 2019;28:2358–2372.e6.
Sasaki K, Sugai T, Ishida K, Osakabe M, Amano H, Kimura H, et al. Analysis of cancer-associated fibroblasts and the epithelial-mesenchymal transition in cutaneous basal cell carcinoma, squamous cell carcinoma, and malignant melanoma. Hum Pathol. 2018;79:1–8.
Hernández-Ruiz E, Hernández-Muñoz I, Masferrer E, Ferrándiz-Pulido C, Andrades E, Gimeno J, et al. A myxoid fibrotic reaction pattern is associated with metastatic risk in cutaneous squamous cell carcinoma. Acta Derm Venereol. 2019;99:89–94.
Saenz-Sardà X, Carrato C, Pérez-Roca L, Puig L, Ferrándiz C, Ariza A, et al. Epithelial-to-mesenchymal transition contributes to invasion in squamous cell carcinomas originated from actinic keratosis through the differentiated pathway, whereas proliferation plays a more significant role in the classical pathway. J Eur Acad Dermatol Venereol. 2018;32:581–6.
Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14:611–29.
Du B, Shim JS. Targeting epithelial–Mesenchymal transition (EMT) to overcome drug resistance in cancer. Molecules. 2016. https://doi.org/10.3390/molecules21070965.
Choi HS, Kim Y-K, Yun P-Y. Upregulation of MDR- and EMT-related molecules in cisplatin-resistant human oral squamous cell carcinoma cell lines. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20123034.
Geng S, Guo Y, Wang Q, Li L, Wang J. Cancer stem-like cells enriched with CD29 and CD44 markers exhibit molecular characteristics with epithelial-mesenchymal transition in squamous cell carcinoma. Arch Dermatol Res. 2013;305:35–47.
Xie S-L, Fan S, Zhang S-Y, et al. SOX8 regulates cancer stem-like properties and cisplatin-induced EMT in tongue squamous cell carcinoma by acting on the Wnt/β-catenin pathway. Int J Cancer. 2018;142:1252–65.
Brabletz T, Kalluri R, Nieto MA, Weinberg RA. EMT in cancer. Nat Rev Cancer. 2018;18:128–34.
Toll A, Masferrer E, Hernández-Ruiz ME, Ferrandiz-Pulido C, Yébenes M, Jaka A, et al. Epithelial to mesenchymal transition markers are associated with an increased metastatic risk in primary cutaneous squamous cell carcinomas but are attenuated in lymph node metastases. J Dermatol Sci. 2013;72:93–102.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Maria-Teresa Fernandez-Figueras has received research funding from Almirall, and has received compensation from Almirall for service as consultant. Luis Puig has received research funding from AbbVie, Almirall, Boehringer Ingelheim, Celgene, Janssen, LEO Pharma, Eli Lilly, Novartis, Pfizer, Regeneron, Roche, Sanofi, and UCB, and has received compensation from AbbVie, Almirall, Baxalta, Biogen, Boehringer Ingelheim, Celgene, Fresenius Kabi, Janssen, BIOCAD, LEO Pharma, Eli Lilly, Mylan, Novartis, Pfizer, Regeneron, Roche, Samsung-Bioepis, Sanofi, and UCB for service as a consultant.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical collection on Skin Cancer
Rights and permissions
About this article
Cite this article
Fernandez-Figueras, MT., Puig, L. The Role of Epithelial-to-Mesenchymal Transition in Cutaneous Squamous Cell Carcinoma. Curr. Treat. Options in Oncol. 21, 47 (2020). https://doi.org/10.1007/s11864-020-00735-x
Published:
DOI: https://doi.org/10.1007/s11864-020-00735-x