Opinion statement
Ewing’s sarcoma is an uncompromising tumor of children and young adults. Before the introduction of chemotherapy for Ewing’s sarcoma, nearly all patients succumbed to their disease, even with highly aggressive approaches to local control. The realization that most patients have micrometastatic disease at presentation, and the identification of active chemotherapeutic agents for this tumor, have resulted in significant improvements in patient survival. Modern therapy for Ewing’s sarcoma combines highdose chemotherapy for systemic control of disease, with advanced surgical and/or radiation therapeutic approaches for local control. Current therapy remains imperfect. Despite optimal management, the cure rate for localized disease is only approximately 70%, whereas the cure rate for metastatic disease at presentation is less than 30%. Patients who experience long-term disease-free survival are at risk for significant side effects of therapy, including infertility, limb dysfunction, and an increased risk for second malignancies. More effective and less toxic therapies are needed. This report presents an overview of dysregulated molecular pathways in Ewing’s sarcoma and highlights the possibility that they may serve as therapeutic targets for the disease. Although a great deal of additional investigation is required before most of these approaches can be assessed in the clinic, we think that these potential new targets offer a great deal of hope for patients with Ewing’s sarcoma.
Similar content being viewed by others
References and Recommended Reading
Arndt CA, Crist CM: Common musculoskeletal tumors of childhood and adolescence. N Engl J Med 1999, 341: 342–352.
Rodriguez-Galindo C, Spunt SL, Pappo AS: Treatment of Ewing sarcoma family of tumors:current status and outlook for the future. Med Pediatr Oncol 2003, 40: 276–287.
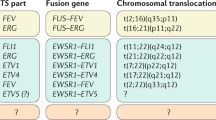
Ladanyi M: EWS-FLI1 and Ewing’s sarcoma:recent molecular data and new insights. Cancer Biol Ther 2002, 1: 330–336.
May WA, Gishizky ML, Lessnick SL, et al.: Ewing sarcoma 11;22 translocation produces a chimeric transcription factor that requires the DNA-binding domain encoded by FLI1 for transformation. Proc Natl Acad Sci U S A 1993, 90: 5752–5756.
Dellatre O, Zucman J, Plougastel B, et al.: Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature 1992, 359: 162–165. This study describes the initial identification of the EWS/FLI fusion oncoprotein.
Ohno T, Ouchida M, Lee L, et al.: The EWS gene, involved in Ewing family of tumors, malignant melanoma of soft parts and desmoplastic small round cell tumors, codes for an RNA binding protein with novel regulatory domains. Oncogene 1994, 9: 3087–3097.
Olsen RJ, Hinrichs SH: Phosphorylation of the EWS IQ domain regulates transcriptional activity of the EWS/ ATF1 and EWS/FLI1 fusion proteins. Oncogene 2001, 20: 1756–1764.
Matsuoka Y, Shibata S, Yasuhara N, Yoneda Y: Identification of Ewing’s sarcoma gene product as a glycoprotein using a monoclonal antibody that recognizes an immunodeterminant containing O-linked Nacetylglucosamine moiety. Hybrid Hybridomics 2001, 21: 233–236.
Belyanskaya LL, Gehrig PM, Gehring H: Exposure on cell surface and extensive arginine methylation of ewing sarcoma (EWS) protein. J Biol Chem 2001, 276: 18681–18687.
Rao VN, Ohno T, Prasad DD, et al.: Analysis of the DNA-binding and transcriptional activation functions of human Fli-1 protein. Oncogene 1993, 8: 2167–2173.
May WA, Lessnick SL, Braun BS, et al.: The Ewing’s sarcoma EWS/FLI-1 fusion gene encodes a more potent transcriptional activator and is a more powerful transforming gene than FLI-1. Mol Cell Biol 1993, 13: 7393–7398. This study demonstrates that the critical difference between EWS/ FLI and wild-type FLI is the presence of a strong transcriptional activation domain in the former. This strong activation domain allows EWS/FLI to function as an oncogenic transcription factor.
Lessnick SL, Braun BS, Denny CT, May WA: Multiple domains mediate transformation by the Ewing’s sarcoma EWS/FLI-1 fusion gene. Oncogene 1995, 10: 423–431.
Jaishankar S, Zhang J, Roussel MF, Baker SJ: Transforming activity of EWS/FLI is not strictly dependent upon DNA-binding activity. Oncogene 1999, 18: 5592–5597.
Welford SM, Hebert SP, Deneen B, et al.: DNA binding domain independent pathways are involved in EWS/ FLI1 mediated oncogenesis. J Biol Chem 2001, 276: 41977–41984.
Ouchida M, Ohno T, Fujimura VN, et al.: Loss of tumorigenicity of Ewing’s sarcoma cells expressing antisense RNA to EWS-fusion transcripts. Oncogene 1995, 11: 1049–1054.
Dohjima T, Lee NS, Li H, et al.: Small interfering RNAs expressed from a Pol III promoter suppress the EWS/ Fli-1 transcript in an Ewing sarcoma cell line. Mol Ther 2003, 7: 811–816.
Liang H, Mao X, Olejniczak ET, et al.: Solution structure of the ets domain of Fli-1 when bound to DNA. Nat Struct Biol 1994, 1: 871–875.
Hagman J, Grosschedl R: An inhibitory carboxyl-terminal domain in Ets-1 and Ets-2 mediates differential binding of ETS family factors to promoter sequences of the mb-1 gene. Proc Natl Acad Sci U S A 1992, 89: 8889–8893.
Stegmaier K, Ross KN, Colavito SA, et al.: Gene expression-based high-throughput screening (GE-HTS) and application to leukemia differentiation. Nat Genet 2004, 36: 257–263.
Braun BS, Frieden R, Lessnick SL, et al.: Identification of target genes for the Ewing’s sarcoma EWS/FLI fusion protein by representational difference analysis. Mol Cell Biol 1995, 15: 4623–4630.
May WA, Arvand A, Thompson AD, et al.: EWS/FLI1-induced manic fringe renders NIH 3T3 cells tumorigenic. Nat Genet 1997, 17: 495–497.
Thompson AD, Braun BS, Arvand A, et al.: EAT-2 is a novel SH2 domain containing protein that is up regulated by Ewing’s sarcoma EWS/FLI1 fusion gene. Oncogene 1996, 13: 2649–2658.
Im YH, Kim HT, Lee C, et al.: EWS-FLI1, EWS-ERG, and EWS-ETV1 oncoproteins of Ewing tumor family all suppress transcription of transforming growth factor beta type II receptor gene. Cancer Res 2000, 60: 1536–1540.
Arvand A, Bastians H, Welford SM, et al.: EWS/FLI1 up regulates mE2-C, a cyclin-selective ubiquitin conjugating enzyme involved in cyclin B destruction. Oncogene 1998, 17: 2039–2045.
Arvand A, Welford SM, Teitell MA, Denny CT: The COOH-terminal domain of FLI-1 is necessary for full tumorigenesis and transcriptional modulation by EWS/FLI-1. Cancer Res 2001, 61: 5311–5317.
Khan J, Wei JS, Ringner M, et al.: Classification and diagnostic prediction of cancers using gene expression profiling and artificial neural networks. Nat Med 2001, 7: 673–679. This study is the first description of the differences in gene expression pattern between various pediatric small round blue cell tumors, including Ewing’s sarcoma.
Lessnick SL, Dacwag CS, Golub TR: The Ewing’s sarcoma oncoprotein EWS/FLI induces a p53-dependent growth arrest in primary human fibroblasts. Cancer Cell 2002, 1: 393–401. This study, with the study be Prieur et al. [28], describes the gene targets of EWS/FLI using microarray technology.
Prieur A, Tirode F, Cohen P, Delattre O: EWS/FLI-1 silencing and gene profiling of Ewing cells reveal downstream oncogenic pathways and a crucial role for repression of insulin-like growth factor binding protein 3. Mol Cell Biol 2004, 24: 7275–7283. This study, with the study by Lessnick et al. [27], describes the gene targets of EWS/FLI using microarray technology.
Bailly RA, Bosselut R, Zucman J, et al.: DNA-binding and transcriptional activation properties of the EWS-FLI-1 fusion protein resulting from the t(11;22) translocation in Ewing sarcoma. Mol Cell Biol 1994, 14: 3230–3241.
Dauphinot L, De Oliveira C, Melot T, et al.: Analysis of the expression of cell cycle regulators in Ewing cell lines:EWS-FLI-1 modulates p57KIP2 and c-Myc expression. Oncogene 2001, 20: 3258–3265.
Hahm KB, Cho K, Lee C, et al.: Repression of the gene encoding the TGF-beta type II receptor is a major target of the EWS-FLI1 oncoprotein. Nat Genet 1999, 23: 222–227.
Mao B, Wu W, Li Y, et al.: LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature 2001, 411: 321–325.
Uren A, Wolf V, Sun VF, et al.: Wnt/Frizzled signaling in Ewing sarcoma. Pediatr Blood Cancer 2004, 43: 243–249.
Druker BJ, Talpaz M, Resta DJ, et al.: Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 2001, 344: 1031–1037.
Yee D, Favoni RE, Lebovic GS, et al.: Insulin-like growth factor I expression by tumors of neuroectodermal origin with the t(11;22) chromosomal translocation. A potential autocrine growth factor. J Clin Invest 1990, 86: 1806–1814.
Toretsky JA, Kalebic T, Blakesley V, et al.: The insulin-like growth factor-I receptor is required for EWS/FLI-1 transformation of fibroblasts. J Biol Chem 1997, 272: 30822–30827.
Mitsiades CS, Mitsiades NS, McMullan CJ, et al.: Inhibition of the insulin-like growth factor receptor-1 tyrosine kinase activity as a therapeutic strategy for multiple myeloma, other hematologic malignancies, and solid tumors. Cancer Cell 2004, 5: 221–230.
Zwerner JP, May WA: PDGF-C is an EWS/FLI induced transforming growth factor in Ewing family tumors. Oncogene 2001, 20: 626–633.
Zwerner JP, May WA: Dominant negative PDGF-C inhibits growth of Ewing family tumor cell lines. Oncogene 2002, 21: 3847–3854.
Merchant MS, Woo CW, Mackall CL, Thiele CJ: Potential use of imatinib in Ewing’s sarcoma:evidence for in vitro and in vivo activity. J Natl Cancer Inst 2002, 94: 1673–1679.
Hotfilder M, Lanvers C, Jurgens H, et al.: c-KIT-expressing Ewing tumour cells are insensitive to imatinib mesylate (STI571). Cancer Chemother Pharmacol 2002, 50: 167–169.
Uren A, Merchant MS, Sun CJ, et al.: Beta-platelet-derived growth factor receptor mediates motility and growth of Ewing’s sarcoma cells. Oncogene 2003, 22: 2334–2342.
Sturla LM, Westwood G, Selby PJ, et al.: Induction of cell death by basic fibroblast growth factor in Ewing’s sarcoma. Cancer Res 2000, 60: 6160–6170.
Sohn HW, Choi EY, Kim SH, et al.: Engagement of CD99 induces apoptosis through a calcineurin-independent pathway in Ewing’s sarcoma cells. Am J Pathol 1998, 153: 1937–1945.
Silvany RE, Eliazer S, Wolff NC, Ilaria RL Jr: Interference with the constitutive activation of ERK1 and ERK2 impairs EWS/FLI-1-dependent transformation. Oncogene 2000, 19: 4523–4530.
Toretsky JA, Thakar M, Eskenazi AE, Frantz CN: Phosphoinositide 3-hydroxide kinase blockade enhances apoptosis in the Ewing’s sarcoma family of tumors. Cancer Res 1999, 59: 5745–5750.
Huang HY, Illei PB, Zhao Z, et al.: Ewing sarcomas with p53 mutation or p16/p14ARF homozygous deletion:a highly lethal subset associated with poor chemoresponse. J Clin Oncol 2005, 23: 548–558. This study demonstrates the utility of molecular markers, including p53 and RB pathway status, as important prognostic factors in Ewing’s sarcoma.
Shapiro GI: Preclinical and clinical development of the cyclin-dependent kinase inhibitor flavopiridol. Clin Cancer Res 2004, 10: 4270s-4275s.
Bischoff JR, Kirn DH, Williams A, et al.: An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science 1996, 274: 373–376.
McCormick F: Cancer-specific viruses and the development of ONYX-015. Cancer Biol Ther 2003, 2: S157-S160.
Zhou Z, Zhou RR, Guan H, et al.: E1A gene therapy inhibits angiogenesis in a Ewing’s sarcoma animal model. Mol Cancer Ther 2003, 2: 1313–1319.
Pavlakovic H, Von Schutz V, Rossler J, et al.: Quantification of angiogenesis stimulators in children with solid malignancies. Int J Cancer 2001, 92: 756–760.
Holzer G, Obermair A, Koschat M, et al.: Concentration of vascular endothelial growth factor (VEGF) in the serum of patients with malignant bone tumors. Med Pediatr Oncol 2001, 36: 601–604.
Fuchs B, Inwards CY, Janknecht R: Vascular endothelial growth factor expression is up-regulated by EWS-ETS oncoproteins and Sp1 and may represent an independent predictor of survival in Ewing’s sarcoma. Clin Cancer Res 2004, 10: 1344–1353.
Bolontrade MF, Zhou RR, Kleinerman ES: Vasculogenesis plays a role in the growth of Ewing’s sarcoma in vivo. Clin Cancer Res 2002, 8: 3622–3627.
Kovar H: Ewing’s sarcoma and peripheral primitive neuroectodermal tumors after their genetic union. Curr Opin Oncol 1998, 10: 334–342.
Cavenzzana AO, Miser JS, Jefferson J, Triche TJ: Experimental evidence for a neural origin of Ewing’s sarcoma of bone. Am J Pathol 1987, 127: 507–518.
Knezevich SR, Hendson G, Mathers JA, et al.: Absence of detectable EWS/FLI1 expression after therapy-induced neural differentiation in Ewing sarcoma. Hum Pathol 1998, 29: 289–294.
Collini P, Mezzelani A, Modena P, et al.: Evidence of neural differentiation in a case of post-therapy primitive neuroectodermal tumor/Ewing sarcoma of bone. Am J Surg Pathol 2003, 27: 1161–1166.
Reynolds CP: Detection and treatment of minimal residual disease in high-risk neuroblastoma. Pediatr Transplant 2004, 8(Suppl5): 56–66.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McAllister, N.R., Lessnick, S.L. The Potential for molecular therapeutic targets in Ewing’s sarcoma. Curr. Treat. Options in Oncol. 6, 461–471 (2005). https://doi.org/10.1007/s11864-005-0025-y
Issue Date:
DOI: https://doi.org/10.1007/s11864-005-0025-y