Opinion statement
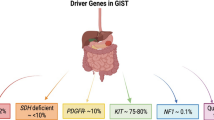
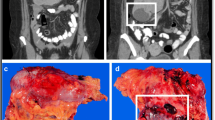
Gastrointestinal stromal tumors (GIST) represent the most common type of mesenchymal malignancy in the gastrointestinal tract. With the discovery of uncontrolled KIT tyrosine kinase signaling as a critical component in the pathogenesis of this disease, the diagnostic and treatment options for patients with GIST have evolved rapidly. Pathology review by an experienced pathologist is critical to the classification of this disease. Expert and definitive surgery remains the mainstay of treatment in patients with localized, resectable disease. Imatinib mesylate has been shown to be the first successful systemic therapy for patients with metastatic or unresectable disease and has revolutionized the treatment of this often rapidly progressive and fatal disease. Ongoing studies are evaluating the role of imatinib in the preoperative and postsurgical adjuvant settings. Although resistance to imatinib will appear over time, there is reason for optimism that the mechanisms of resistance will be identified and eventually overcome. The application of molecular understanding of GIST to the development of a selective, scientifically rational therapy is a classic example of multidisciplinary translational oncology research.
Similar content being viewed by others
References and Recommended Reading
Mazur MT, Clark HB: Gastric stromal tumors: reappraisal of histogenesis. Am J Surg Pathol 1983, 7:507–519.
Lauwers GY, Erlandson RA, Casper ES, et al.: Gastrointestinal autonomic nerve tumors: a clinicopathological, immunohistochemical, and ultrastructural study of 12 cases. Am J Surg Pathol 1993, 17:887–897.
Miettinen M, Virolainen M, Maarit Sarlomo R: Gastrointestinal stromal tumors-value of CD34 antigen in their identification and separation from true leiomyomas and schwannomas. Am J Surg Pathol 1995, 19:207–216.
Miettinen M, Fanburg-Smith JC, Virolainen M, et al.: Epithelioid sarcoma: an immunohistochemical analysis of 112 classical and variant cases and a discussion of the differential diagnosis. Hum Pathol 1999, 30:934–942.
Hirota S, Isozaki K, Moriyama Y, et al.: Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998, 279:577–580. The first description of the c-KIT mutation in GIST.
Rubin BP, Singer S, Tsao C, et al.: KIT activation is a ubiquitous feature of gastrointestinal stromal tumors. Cancer Res 2001, 61:8118–8121.
DeMatteo RP, Lewis JJ, Leung D, et al.: Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000, 231:51–58. The largest surgical series published on GIST.
Miettinen M, Lasota J: Gastrointestinal stromal tumors—definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch 2001, 438:1–12.
Emory TS, Sobin LH, Lukes L, et al.: Prognosis of gastrointestinal smooth-muscle (stromal) tumors: dependence on anatomic site. Am J Surg Pathol 1999, 23:82–87.
Fletcher CD, Berman JJ, Corless C, et al.: Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol 2002, 33:459–465. This paper is a consensus document based on a GIST workshop held at the National Institutes of Health in 2001. The workshop was attended by many experts in the field, and provided an excellent, up-to-date perspective.
Ng EH, Pollock RE, Munsell MF, et al.: Prognostic factors influencing survival in gastrointestinal leiomyosarcomas: implications for surgical management and staging. Ann Surg 1992, 215:68–77.
Goss GA, Merriam P, Manola J, et al.: Clinical and pathological characteristics of gastrointestinal stromal tumors (GIST). 19th Annual Meeting of the American Society of Clinical Oncology, New Orleans, LA; May 2000. Abstract no. 559a.
Druker BJ, Tamura S, Buchdunger E, et al.: Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med 1996, 2:561–566. This landmark paper was the first report on the use of STI-571, designed to inhibit the abl protein tyrosine kinase, in bcr-abl positive CML.
Tuveson DA, Willis NA, Jacks T, et al.: STI571 inactivation of the gastrointestinal stromal tumor c-KIT oncoprotein: biological and clinical implications. Oncogene 2001, 20:5054–5058.
Joensuu H, Roberts PJ, Sarlomo-Rikala M, et al.: Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med 2001, 344:1052–1056. This is a report of the first patient treated with STI-571, who had a dramatic response to treatment.
Demetri GD, von Mehren M, Blanke CD, et al.: Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002, 347:472–480. This randomized phase II study on the use of imatinib in GIST is the largest and most important published study, and provides us with the most comprehensive clinical perspective.
Van den Abbeele A, Badawi RD, Cliché JP, et al.: 18F-FDG-PET predicts response to imatinib mesylate (Gleevec) in patients with advanced gastrointestinal stromal tumors (GIST). 21st Annual Meeting of the American Society of Clinical Oncology, Orlando, Florida; May 15-21, 2002. Abstract no. 1610.
Demetri GD, Rankin C, Fletcher CD, et al.: Phase III dose-randomized study of imatinib mesylate (Gleevec, STI571) for GIST: Intergroup S0033 early results. 21st Annual Meeting of the American Society of Clinical Oncology, Orlando, Florida; May 15-21, 2002. Abstract no. 1651.
Casali PG, Verweij J, Zalcberg J, et al.: Imatinib (Glivec) 400 vs 800 mg daily in patients with gastrointestinal stromal tumors (GIST): a randomized phase III trial from the EORTC Soft Tissue and Bone Sarcoma Group, the Italian Sarcoma Group (ISG), and the Australasian Gastro-Intestinal Trials Group. 21st Annual Meeting of the American Society of Clinical Oncology, Orlando, Florida; May 15-21, 2002. Abstract no. 1650.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
George, S., Desai, J. Management of gastrointestinal stromal tumors in the era of tyrosine kinase inhibitors. Curr. Treat. Options in Oncol. 3, 489–496 (2002). https://doi.org/10.1007/s11864-002-0068-2
Issue Date:
DOI: https://doi.org/10.1007/s11864-002-0068-2