Abstract
Objectives
To evaluate the frequency, characteristics, and persistence of headache in coronavirus disease-19 (COVID-19) patients who are hospitalised and to determine if there is a link between headache and smell and/or taste dysfunction.
Materials and methods
In April and May 2020, patients who were hospitalised due to COVID-19 and had headache complaints were evaluated by a neurologist. In addition to clinical COVID-19 features, the characteristics and course of the patients’ headaches were evaluated. The patients were contacted by phone 3 months after they were discharged from the hospital to determine the persistence of their symptoms.
Results
Eighty-five patients were included in the study, 54.1% were female; the mean age was 47.5 ± 13.9 years (between the ages of 21 and 84). Fifty-four patients (65.3%) presented with smell and/or taste dysfunction, and 14 patients (n = 14, 25.9%) still reported that dysfunction 3 months later. Moreover, 17 (20%) still had headaches 3 months after being discharged from the hospital. Persistent smell/taste disorders were significantly (p < 0.001) more frequent in patients with persistent headaches (59%) compared to those without (6%) (p < 0.001).
Conclusion
In this prospective study in COVID-19 patients presenting with headache upon admission, a correlation between persistent headache 3 months after discharge and persistent smell/taste dysfunction was found that could point to common underlying pathophysiology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
COVID-19 was identified as a global epidemic by the World Health Organization (WHO) in early March 2020 [1]. Although COVID-19 symptoms are mostly respiratory, symptoms and consequences in the central and peripheral neurological systems, such as anosmia, ageusia, and headache, are increasingly being reported [2]. Virus-induced cell damage, immunological processes, and hypoxia may all play a role in these consequences [3]. The COVID-19 pandemic is thought to have caused a fivefold increase in the incidence of headaches in the areas affected by this disease [4]. According to a recent meta-analysis, the prevalence of headache is 10.9% in COVID-19 patients, with a significant degree of heterogeneity [5]. The symptoms of these headaches are poorly understood. A more common pattern similar to a tension-type headache was identified in an Italian research study that measured neurological symptoms in hospitalised patients [6]. It has been reported that headaches are usually bilateral [7]. In a study conducted in Turkey, it was reported that COVID-19 patients with anosmia/ageusia and gastrointestinal problems were more likely to have bilateral, long-lasting headaches and analgesic resistance, and be male [8]. The olfactory system was impaired in 52.7% of COVID-19 patients, and the gustatory system was impaired in 43.9% [9, 10].
The features, consequences, and temporal evolution of headaches are all unknown [11]. Currently, only few studies on post-COVID-19 headache and only case reports are available [12,13,14].
In their everyday practice, neurologists observe a wide variety of different clinical presentations in patients with post-COVID-19 headaches. It is vital to remember that this terminology (post-COVID-19 headache) can refer to a variety of clinically distinct headaches. This emphasises the significance of paying careful attention when measuring the complexity of post-COVID-19 headaches, as well as the need to learn more about the many pathophysiological mechanisms at play.
The aim of this study is to determine the prevalence and characteristics of headache in hospitalised COVID-19 patients and to identify if there is a link between headache and the symptoms of smell and/or taste dysfunction and persistence of headache and smell and/or taste dysfunction complaints after 3 months.
Materials and method
This research study was conducted in compliance with the Declaration of Helsinki and was approved by Istanbul Zaim University Ethics Committee (Number: 14/08/2020_E.4028). Our study included patients diagnosed in our hospital according to the “COVID-19 diagnosis and treatment protocol guideline” [15] of the Ministry of Health, who had headaches upon admission. The study’s participants gave their written informed consent. Data were collected from patients aged 18 years or older. Patients with a decreased level of consciousness, confusion, or cognitive impairment were excluded.
During the COVID-19 pandemic, all physicians on the hospital clinical staff, regardless of their specialities, provided care for COVID-19 patients. Consequently, neurologists were in charge of keeping track of the patients who were admitted to hospital services. In April and May 2020, patients who were hospitalised due to COVID-19 and had headache complaints were evaluated by a neurologist. For data collection, a semi-structured questionnaire was used, which included sociodemographic data, information on the occurrence and characteristics of headache during COVID-19 and associated symptoms, and whether there was the smell and/or taste dysfunction (hyposmia/anosmia and/or hypogeusia/ageusia).
Patients who reported COVID-19-related headaches were asked about previous headaches and their characteristics. Patients who had previously experienced headaches were asked if the headache linked with COVID-19 was comparable to or different from previous headaches. The third version of the International Classification of Headache Disorders (ICHD-3) was used to classify the previous headaches reported by these individuals [16]. If headaches met criteria A through C1 for “headache attributed to systemic viral infection”, they were classified as COVID-19 related (9.2.2) [16].
For patients with headaches, we measured the time between the beginning of the first COVID-19 symptoms and the commencement of the headache. Patients were asked about topography, such as whether the headache was bilateral or unilateral, and if it was centred in the frontal, temporal, parietal, or occipital regions. On a 0–10 numeric rating scale (NRS) (0: no pain, 10: worst possible pain), we investigated the quality and severity of the pain. Mild pain (NRS: 1–3), moderate pain (NRS: 4–6), or severe pain (NRS: 7–10) were used to classify pain intensity [16]. The patients were all asked about photophobia, phonophobia, osmophobia, nausea, or/and vomiting. We also asked about the worsening of the headache by moving or coughing.
Ophthalmoscopy and olfactory and taste tests were not done during the physical examination to reduce the danger of contaminating the evaluator. There were no neurological symptoms or evidence of viral meningoencephalitis. None of the patients reported having a runny nose or nasal congestion, which are common signs of viral respiratory infections.
The patients were contacted by phone 3 months after they were discharged from the hospital. We followed up with patients over the phone to assess the headache persistence and characteristics, as well as the presence of smell and/or taste dysfunction (hyposmia/anosmia and/or hypogeusia/ageusia).
Statistical analysis
Statistical analyses were performed using the SPSS version 25.0 software program. We used frequency and percentage to describe the qualitative variables. We used mean and standard deviation (SD) to describe the continuous variables if the distribution was normal, or median and interquartile range (IQR) if the distribution was not normal. The conformity of the variables to the normal distribution was examined by histogram graphics and the Kolmogorov–Smirnov test. Categorical variables were compared with the Pearson Chi-square test. The Mann–Whitney U test was used to evaluate the non-normally distributed (nonparametric) variables between two groups. Cases with p-value < 0.05 are statistically significant.
Results
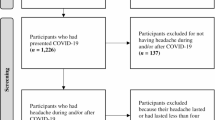
During the research period, we included 85 patients who were admitted to our hospital with a diagnosis of COVID-19 in April and May 2020 and who were cooperative in answering every question asked and consented to participate in the study.
There were no symptoms of decreased consciousness, meningitis, or focal neurologic abnormalities in any of the individuals. Because there were no warning signals of COVID-19-related neurological problems, no patients underwent brain computed tomography (CT), magnetic resonance imaging (MRI), or cerebrospinal fluid analysis.
In our study, the average age of the patients was 47.5 ± 13.9 years (age range: 21–84), and 54.1% (46/85) were female. The sociodemographic data of the patients and the characteristics of the headaches are listed in Table 1.
The headache appeared in 81 (95.2%) of the patients already on the first symptom day. Overall 22 (25.8%) of our patients reported a history of headaches before COVID-19. Eleven had a tension headache, 8 had a migraine, and 3 were diagnosed with a secondary headache according to ICHD-3 criteria. All the COVID-19 patients with previous headaches stated that their new developing headaches during the infection period were distinct from their normal headaches. Three months after hospital discharge, 17 (20%) individuals were still suffering from headaches at the post-discharge examination.
Headache phenotype
The headache topography was bilateral in 76 (89.4%) patients and hemicranial in 9 (10.6%) patients. Frontal headaches were reported in 30 (35.2%) patients. The pain was described as pressing/tightening in 33 (38.8%) of the patients, pulsating in 47 (55.2%) patients.
The headaches were mild in 10 (11.7%) patients, moderate in 50 (58.8%) patients, and severe in 25 (29.4%) patients. In 14 (17.7%) patients, the headaches were completely resolved with analgesics.
The headache was worsened by movement in 19 (22.3%) patients. Coughing made the headache worse in 9 (10.5%) patients. Nausea and/or vomiting was described by 52 (61.1%) patients.
Smell and/or taste dysfunction (hyposmia/anosmia and/or hypogeusia/ageusia)
Fifty-four (63.5%) patients reported smell and/or taste dysfunction (hyposmia/anosmia and/or hypogeusia/ageusia). Fourteen (n = 14, 25.9%) patients still reported this dysfunction 3 months after being discharged from the hospital. There was no qualitative difference based on gender (chi-square test; p = 0.209).
Persistent headache
Seventeen (20%) patients still had headaches 3 months after hospital discharge. We looked at individuals with a persistent headache after 3 months and discovered that 76.4% (13/17) had never previously experienced a recurring headache. Then, after 3 months, we compared individuals with chronic headaches to those who were headache-free. There is no significant difference between those who have persistent headaches and those who do not, in terms of previous headache (p = 0.804), gender (p = 0.231), age (p = 0.191), and a number of hospitalisation days (p = 0.363). The number of patients who have persistent headaches and with persistent smell and/or taste dysfunction was higher than the number of patients in which headaches did not continue (p < 0.001) (Table 2).
Discussion
Our study primarily aimed to describe the characteristics and evolution of headaches associated with hospitalised COVID-19 patients and to relate it to smell/taste dysfunction. It also aimed to form a hypothesis about the underlying pathophysiological mechanisms of these conditions in COVID-19 patients.
In our study, we observed that the majority of COVID-19 hospitalised patients had moderate/severe headaches, which began at the outset of COVID-19 symptoms. Headaches were usually bilateral in the frontal region, with a pulsating quality, and often accompanied by nausea or/and vomiting and poor response to analgesics; these findings are similar to those reported in earlier studies [8, 9]. Headache was not associated with a personal history of migraine.
A recent study from Spain [9] identified different phenotypes based on headache characteristics in COVID-19 and the presence of a personal history of primary headache. According to a meta-analysis, headaches were recorded in 11–13% of hospitalised COVID-19 patients and 6–10% of symptomatic individuals. It commonly manifests as a moderate-to-severe bilateral headache with pulsating or pressing symptoms in the temporoparietal, forehead or periorbital areas. It usually has a “sudden to slow onset” and has a poor response to conventional analgesics [11].
According to our research, COVID-19-related headaches often start when the other COVID-19 symptoms begin. Headache was identified as the initial COVID-19 symptom in a recent case report [17]. According to a recent assessment, most neurological symptoms are expected to appear in the early stages of the COVID-19, with a median of 1–2 days [5].
In COVID-19, the presence of headache is significantly associated with other cranial symptoms, such as anosmia/ageusia [11]. Specifically, these symptoms have been frequently reported in case reports of patients with headaches during COVID-19 [18].
Smell and/or taste dysfunction was also highly prevalent; it was found in 63.5% of the patients in our study; in 16.4% of patients, it was still present 3 months after being discharged from the hospital.
In a large multi-centre European sample of patients with mild-to-moderate COVID-19, olfactory and gustatory impairment were found in 85.6% and 88.8% of the cases, respectively [19]. Olfactory impairment was mostly self-limiting, whereas gustatory dysfunction lingered in more than 70% of individuals long after their respiratory symptoms had subsided. One study found that, occasionally, anosmia and ageusia were the initial signs presenting before respiratory symptoms [20]. Anosmia was the first symptom in 36% of the participants in a COVID-19 study conducted in Spain [21]. In our study, smell and/or taste dysfunction began with the onset of other COVID-19 symptoms in most of the patients.
The olfactory bulb volume and signal intensity were normal on MRI in a patient with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-related isolated acute anosmia [22]. A patient with anosmia, dysgeusia, and cortical hyperintensity in the right gyrus rectus, as well as modest hyperintensity in the olfactory bulbs, was recently described, which was consistent with viral brain invasion [23].
Another research study performed MRIs on four patients with headaches [24]; employing a specialised approach for processing MRI, they identified five instances of COVID-19 (three with anosmia) with alterations in the olfactory bulb, indicative of microbleeding or aberrant enhancement.
In [19], 72.6% of the individuals with COVID-19 regained olfactory function during the first 8 days, suggesting that anosmia is mostly a transient condition. In one study, hyposmia/anosmia persisted in 19% of the patients after 100 days [25].
In terms of headache progression, we found that, after 3 months, 20% of the patients in our study had a persistent disabling daily headache, with a poor acute therapeutic response. Furthermore, the headache was an early prodromal symptom of COVID-19 in a significant number of these individuals. We found that males and patients with no previous history of headaches were more likely to have persistent headaches.
While SARS-CoV-2 harms the nervous system, the mechanisms are yet unknown [11]. Underlying this headache type is a meningeal peripheral sensitisation and consequently activation of the trigeminovascular system, given that headache produced by COVID-19 can mimic migraine in persons who have never had it [26]. Among the several pathways that might lead to the activation of the trigeminovascular system, it is important to mention that a link between headache and anosmia/ageusia was found to be a unique COVID-19 symptom [20, 27]. SARS-CoV-2 may have neurotropic properties, allowing it to infiltrate peripheral nerve terminals and enter the central nervous system via transsynaptic pathways [28,29,30]. Passive diffusion and axonal transport have been proven to be the viral entry point for COVID-19 from the nasal cavity to the olfactory bulb, extending to the brainstem through the piriform cortex [31]. Concomitant anosmia inside the nasal cavity may imply pathogen-mediated peripheral activation of the trigeminovascular system, operating not only on the specialised olfactory epithelium but also on the trigeminal branches that exist at this level or through olfactory-trigeminal interactions [32].
Endothelitis, like other pathophysiological processes, might have a role in COVID-19 [33], since inflammatory responses could be triggered by the interaction between the virus with its receptor, angiotensin-converting enzyme 2 (ACE2), which is found in the endothelium of blood vessels.
SARS-CoV-2 can spread via the bloodstream to the endothelium of small vessels in the cerebral circulation [34], where ACE2 is expressed [35]. While SARS-CoV-1 and MERS-CoV have been proven to infect the central nervous system by the transsynaptic spread in transgenic mouse models [30], it is unknown if SARS-CoV-2 infects the brain through the olfactory tract in the same way. According to previous research, ACE2 was found to be expressed in the olfactory epithelium but not in the olfactory sensory neurons. Thus, rather than olfactory neuronal destruction, clinical anosmia is most likely caused by SARS-CoV-2-induced olfactory epithelial cell damage [36, 37].
According to a meta-analysis, the prevalence of post-COVID-19 headache ranges from 8 to 15% within the first 6 months following acute infection [38]. Significantly, up to half of these patients had no previous history of recurrent headaches, which might indicate the start of a “new daily” headache. In headache clinics, new daily persistent headache (NDPH) has become one of the most difficult illnesses to treat. A viral infection is the most common triggering event for NDPH, occurring in up to 30% of patients [39,40,41]. Men and women are both affected by the viral trigger [42]. NDPH has been recorded as the main symptom of acute SARS-CoV-2 infection and as a common lingering complaint of the extended illness in individuals with even moderate instances of SARS-CoV-2 infection [19, 43, 44].
The rate of smell and taste dysfunction was high in our study. The persistence rate of smell and taste dysfunction was compared between patients who had persistent headaches and those who did not. After 3 months, the rate of smell/taste dysfunction in patients whose headache persisted was significantly higher than in those whose headache did not persist. This might provide evidence of the persistence of trigeminovascular system susceptibility leading to headache persistence and chronification. In our study, a significant number of individuals without a personal history of the previous headache reported chronic headache; this finding is similar to the results reported in (32). This shows that, in our clinical practice, this postinfectious aetiology may be an underdiagnosed source of NDPH.
This study has some limitations. The sample size was small, many of the questionnaire questions were based on patient recollection, the evaluation of symptoms was subjective, and neither olfactory tests nor taste tests were done to objectively verify anosmia and hyposmia. Moreover, we cannot rule out the possibility that the research could not detect modest variations between the groups, since we did not compute the sample size. While presenting with respiratory symptoms, the patients were only seen once. Then, after 3 months, the patients were contacted by phone to determine the persistence of headache and/or smell/taste dysfunction.
Other causes of secondary headaches (such as meningitis, encephalitis, and cerebrovascular disorders), which may be consequences of COVID-19, were not ruled out using neuroimaging, cerebrospinal fluid testing or ophthalmoscopy. However, the study participants did not have any meningeal or focal symptoms, confusional states, or a reduced level of awareness, making these problems less likely.
Only inpatients were included in this investigation, which was done in a single facility. Because this reduces the study’s capacity to generalise its results, extending the findings to individuals with moderate forms of the condition who do not require hospitalisation should be done with caution.
For effective care, it is necessary to assess how COVID-19 affects individuals with a prior headache problem. All of the participants in our study who had a history of headache problems experienced pain that was different from what they were used to. COVID-19 education for patients in a clinic presenting with a headache should emphasize that COVID-19 can begin with a headache that is distinct from what is generally experienced before further COVID-19-related symptoms appear. Finally, this study is only one among other prospective studies on headache and COVID-19 that are now underway. However, in the event of future new COVID-19 peaks, the information provided in this article will be beneficial to clinicians.
References
Huang C, Wang Y, Li X et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223):497–506
Ellul MA, Benjamin L, Singh B et al (2020) Neurological associations of COVID-19. Lancet Neurol 19(9):767–783
Wu Y, Xu X, Chen Z et al (2020) Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun 87:18–22
Lippi G, Mattiuzzi C, Bovo C et al (2020) Headache is an important symptom in patients with coronavirus disease 2019 (COVID-19). Diagnosis (Berl) .18;7(4):409-411
Pinzon RT, Wijaya VO, Buana RB et al (2020) Neurologic characteristics in coronavirus disease 2019(COVID-19): a systematic review and meta-analysis. Front Neurol 11:565
Vacchiano V, Riguzzi P, Volpi L et al (2020) Early neurological manifestations of hospitalized COVID-19 patients. Neurol Sci 41:2029–2031
Karadas O, Ozturk B, Sonkaya AR (2020) A prospective clinical study of detailed neurological manifestations in patients with COVID-19. Neurol Sci 41:1991–1995
Uygun Ö, Ertaş M, Ekizoğlu E et al (2020) Headache characteristics in COVID-19 pandemic –a survey study. J Headache Pain 21:121
Rocha-Filho P, Magalhães J (2020) Headache associated with COVID-19: frequency, characteristics and association with anosmia and ageusia. Cephalalgia 40(13):1443–1451
Tong JY, Wong A, Zhu D et al (2020) The prevalence of olfactory and gustatory dysfunction in COVID-19 patients: a systematic review and meta-analysis. Otolaryngol – Head Neck Surg 163:3–11
Bolay H, Gül A, Baykan B (2020) COVID‐19 is a real headache! Headache. 60(7):1415-1421
Belvis R (2020) Headaches during COVID-19: my clinical case and review of the literature. Headache 60:1422–1426
Dono F, Consoli S, Evangelista G et al (2021) New daily persistent headache after SARS-CoV-2 infection: a report of two cases. Neurol Sci 42(10):3965–3968
Sampaio Rocha-Filho PA, Voss L (2020) Persistent headache and persistent anosmia associated with COVID-19. Headache 60:1797–1799
Sağlık Bakanlığı TC (2020) COVID-19 Erişkin Hasta Yönetimi ve Tedavisi Rehberi.
Headache Classification Committee of the International Headache Society.(2018) In The International Classification of Headache Disorders, 3rd edn. Cephalalgia 38:1–211
Singh J, Ali A (2020) Headache as the presenting symptom in 2 patients with COVID-19 and a history of migraine: 2 case reports. Headache 60:1773–1776
Caronna E, Alpuente A, Torres-Ferrus M, Pozo-Rosich P (2021) Toward a better understanding of persistent headache after mild COVID-19: three migraine-like yet distinct scenarios. Headache 61(8):1277–1280
Lechien JR, Chiesa-Estomba CM, De Siati DR et al (2020) Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Oto-Rhino-Laryngology 277:2251–2261
Mao L, Jin H, Wang M et al (2020) Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 77:683–690
Whittaker A, Anson M, Harky A (2020) Neurological manifestations of COVID-19: a systematic review and current update. Acta Neurol Scand 142:14–22
Galougahi MK, Ghorbani J, Bakhshayeshkaram M et al (2020) Olfactory bulb magnetic resonance imaging in SARS-CoV-2-induced anosmia: the first report. Acad Radiol 27(6):892–893
Politi L, Salsano E, Grimaldi M (2020) Magnetic resonance imaging alteration of the brain in a patient with coronavirus disease 2019 (COVID-19) and anosmia. JAMA Neurol 77:1028–1029
Aragão MDFVV, de Leal MC, Cartaxo Filho OQ et al (2020) Anosmia in COVID-19 associated with ınjury to the olfactory bulbs evident on MRI. AJNR Am J Neuroradiol . 41(9):1703–1706
Sonnweber T, Sahanic S, Pizzini A et al (2020) Cardiopulmonary recovery after COVID-19 – an observational prospective multi-center trial. Eur Respir J 57(4):2003481
Ramachandran R (2018) Neurogenic inflammation and its role in migraine. Semin Immunopathol 40:301–314
Vaira LA, Salzano G, Deiana G et al (2020) Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope 130:1787
Li YC, Bai WZ, Hashikawa T (2020) The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol 92:552–555
Zubair AS, McAlpine LS, Gardin T et al (2020) Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol 77:1018–1027
Politi L, Salsano E, Grimaldi M (2020) Magnetic resonance imaging alteration of the brain in a patient with coronavirus disease 2019 (COVID-19) and anosmia. JAMA Neurol 77:1028–1029
Desforges M, Le Coupanec A, Dubeau P et al (2020) Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses 12(1):14
Caronna E, Ballve A, Llaurado A et al (2020) Headache: a striking prodromal and persistent symptom, predictive of COVID-19 clinical evolution. Cephalalgia 40:1410–1421
Varga Z, Flammer AJ, Steiger P et al (2020) Endothelial cell infection and endotheliitis in COVID-19. Lancet 395:1417–1418
Hamming I, Timens W, Bulthuis MLC et al (2004) Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203:631–637
Baig AM, Khaleeq A, Ali U et al (2020) Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 11:995–998
Brann D, Tsukahara T, Weinreb C et al (2020) Non-neural expression of SARS-CoV-2 entry genes in the olfactory epithelium suggests mechanisms underlying anosmia in COVID-19 patients.Sci.Adv 31;6(31):eabc5801
Fodoulian L, Tuberosa J, Rossier D et al (2020) SARS-CoV-2 Receptor andentry genes are expressed by sustentacular cells in the human olfactory neuroepithelium. iScience. 18;23(12):101839
Fernandez-de-Las-Penas C, Navarro-Santana M, Gomez-Mayordomo V et al (2021) Headache as an acute and post-COVID-19 symptom in COVID-19 survivors: a meta-analysis of the current literature. Eur J Neurol 28:3820–3825
Li D, Rozen TD (2002) The clinical characteristics of new daily persistent headache. Cephalalgia 22:66–69
Robbins MS, Grosberg BM, Napchan U et al (2010) Clinical and prognostic subforms of new daily-persistent headache. Neurology 74:1358–1364
Prakash S, Saini S, Rana KR et al (2012) Refining clinical features and therapeutic options of new daily persistent headache: a retrospective study of 63 patients in India. J Headache Pain 13:477–485
Rozen TD (2016) Triggering events and new daily persistent headache: age and gender differences and insights on pathogenesis. Headache 56:164–173
Xu X-W, Wu X-X, Jiang X-G (2020) Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 19;368:m606.
Tenforde MW, Kim SS, Lindsell CJ et al (2020) Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network – United States, March–June 2020. MMWR Morb Mortal Wkly Rep. 31;69(30):993–998
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This research study was conducted in compliance with the Declaration of Helsinki and was approved by Istanbul Zaim University Ethics Committee (Number: 14/08/2020_E.4028).
Consent to participate
The study’s participants gave their written informed consent.
Conflict of interest
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akıncı, T. Post-discharge persistent headache and smell or taste dysfunction after hospitalisation for COVID-19: a single-centre study. Ir J Med Sci 192, 369–375 (2023). https://doi.org/10.1007/s11845-022-02980-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-022-02980-5