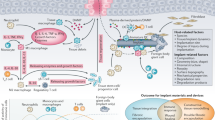
Abstract
This paper aims to give a broad overview of the challenges that are faced in load-bearing medical devices and focuses specifically on the challenges faced in utilizing polymeric materials in such applications. Three specific cases are given in the field of polymeric biomaterials. These cases build in complexity and initiate with examination of the evolution of intravascular catheter design in which the materials, properties, and processing have been optimized to develop a system that can be used in an angioplasty procedure with little concern of clinical failure.
Similar content being viewed by others
References
J.I. Kroschwitz, editor, Polymers: Biomaterials and Medical Applications (New York: Wiley, 1989).
J. Black, Biological Performance of Materials: Fundamentals of Biocompatibility (New York: Marcel Dekker, 1999).
J.B. Park and J.D. Bronzino, Biomaterials: Principals and Applications (Boca Raton, FL: CRC Press, 2003).
B.D. Ratner et al., Biomaterials Science: An Introduction to Materials in Medicine (New York: Academic Press, 1996).
J.B. Park and R.S. Lakes, Biomaterials: An Intro Introduction (New York: Plenum Press, 1992).
D.W. Howie et al., Clin. Orthop. (1993), N. AM. 24:4 571–581.
H.-G. Willert and M. Semlitsch, J. Biomed. Materials Res., 11 (1977), p. 157.
J.L. Gilbert, C.A. Buckley, and J.J. Jacobs, J. Biomed. Res., 27(12) (1994), pp. 1533–1544.
R.J. Young and P.A. Lovell, Introduction to Polymers (London: CRC Press, 1990).
A.H. Matsumoto et al., Cardiovasc. Intervent. Radiol., 16 (1993), p. 135.
C.A. Fontirroche and S. Querns, U.S. patent 5, 820,594 (1998).
S. Gad, Safety Evaluation of Medical Devices (New York: CRC, 2001), pp. 505–536.
S. Bondurant, V.L. Ernster, and R. Herdman, Safety of Silicone Breast Implants (Washington, D.C.: Institute of Medicine National Academy Press, 1999), pp. 253–254.
D.A. Kessler, New Engl. J. Med., 326 (1992), pp. 1713–1715.
L.R. Holmich et al., Plastic and Reconstructive Surgery, 120(7) (2007), pp. 62S–69S.
“Mentor MemoryGel™ Silicone Gel-Filled Breast Implants Product Insert Data Sheet” Physician Labeling Document (Silver Spring, MD: Food and Drug Administration, 2006)
“NIH Consensus Development Conference on Total Knee Replacement” (Bethesda, MD: National Institutes of Health), http://consensus.nih.gov/2003/2003TotalKneeReplacement117html.htm.
Total Hip Replacement, NIH Consensus Statement, 12(5) (1994), pp. 1–31.
H.G. Willert, J. Biomed. Mater. Res., 11(2) (1977), pp. 157–164.
D.W. Howie, J. Arthroplasty, 5(4) (1990), pp. 337–348.
D.O. O’Connor et al., Transactions of 44th Annual Meeting of the Orthopaedic Research Society (Rosemont, IL: Orthopaedic Research Society, 1999), p. 816.
D.A. Baker, A. Bellare, and L. Pruitt, J. Biomedical Materials Research, 66A (2003), pp. 146–154.
S.S. Tower et al., J. Bone Joint Surg. Am., 10 (2007), pp. 2212–2217.
J. Furmanski et al., “Catastrophic Rim Fracture of Four Highly Cross-linked Acetabular Liners” (Paper presented at the Annual Meeting of the Am. Acad. Ortho. Surg., 2008).
W.Y. Shon et al., J. Arthroplasty, 4 (2005), pp. 427–435.
J. Furmanski et al., “In vivo Crack Initiation in Retrieved Cross-linked UHMWPE Acetabular Liners,” Transactions of the 55th Annual Meeting of the Orthopaedic Research Society (Rosemont, IL: Orthopaedic Research Society, 2009).
J. Furmanski, C.M. Rimnac, and L.A. Pruitt, “Brittle Fatigue Crack Propagation of UHMWPE and Its Implications for Total Joint Replacements” (Paper presented at the 14th International Conference on Deformation, Yield and Fracture of Polymers, Kerkrade, The Netherlands, 2009).
J. Furmanski, E. Feest, and L.A. Pruitt, “Static Mode Fatigue of UHMWPE” (Paper presented at the 2nd International Congress on the Mechanics of Biomaterials and Tissues, Kauai, HI, 2007).
J.G. Williams, Fracture Mechanics of Polymers (Chichester, U.K.: Ellis Horwood Ltd., 1984).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pruitt, L., Furmanski, J. Polymeric biomaterials for load-bearing medical devices. JOM 61, 14–20 (2009). https://doi.org/10.1007/s11837-009-0126-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-009-0126-3