Abstract
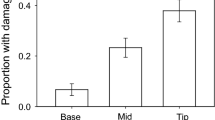
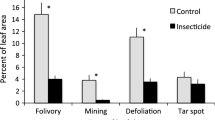
Grasses frequently have unidirectional hairs, prickles, and spines; these leaf features have been hypothesized to move herbivores and their chewing damage away from grass meristems, which are located basally. Observations of chewing damage to two grasses, Andropogon virginicus and Phragmites australis, were consistent with this hypothesis as leaf tips received 10 × and 2 × more damage than bases. Grasshoppers were no more likely to land on leaf tips than bases although they oriented towards the tips after landing. Leaves of A. virginicus that were damaged by chewing herbivores had fewer spines than leaves on the same or neighboring plants that lacked damage. This suggests that herbivores chose less spiny leaves. At a larger spatial scale, plants in neighborhoods favorable for grasshoppers had more spines than plants in less favorable neighborhoods. We found no evidence that marginal spines allowed leaves to shed water more rapidly, a potential alternative benefit. The density of spines on new leaves increased following cues of damage. A. virginicus leaves produced after an adjacent leaf had been clipped with scissors had 13% more spines than new leaves on unclipped plants. Clipping with scissors failed to increase spine density for new P. australis leaves although experimental chewing by caterpillars led to the production of new leaves with 24% more spines than controls. Unchewed new leaves within 20 cm of a chewed neighbor had 13% more spines than controls. Grasses are capable of responding to cues of tissue damage to their own and neighboring leaves, potentially reducing herbivory to meristems.





Similar content being viewed by others
References
Agrawal AA, Hastings AP, Johnson MTJ, Maron JL, Salminen J-P (2012) Insect herbivores drive real-time ecological and evolutionary change in plant populations. Science 338:113–116
Babikova Z, Gilbert L, Bruce TJA, Birkett M, Caulfield JC, Woodcock C, Pickett JA, Johnson D (2013) Underground signals carried through common mycelial networks warn neighboring plants of aphid attack. Ecol Lett 16:835–843
Barton KE (2016) Tougher and thornier: general patterns in the induction of physical defence traits. Funct Ecol 30:181–187
Bell AD (2008) Plant form. An illustrated guide to flowering plant morphology. Timber Press, Portland
Dean JM, Smith AP (1978) Behavioral and morphological adaptations of a tropical plant to high rainfall. Biotropica 10:152–154
Eisner T, Eisner M, Hoebeke ER (1998) When defense backfires: detrimental effect of a plant’s protective trichomes on an insect beneficial to the plant. Proc Natl Acad Sci 95:4410–4414
Engelberth J, Alborn HT, Schmelz EA, Tumlinson JH (2004) Airborne signals prime plants against insect herbivore attack. Proc Natl Acad Sci 101:1781–1785
Feeny P (1976) Plant apparency and chemical defense. Recent Adv Phytochem 10:1–40
Feild TS, Sage JL, Czerniak C, Iles WJD (2005) Hydrothodal leaf teeth of Chloranthus japonicus (Chloranthaceae) prevent guttation-induced flooding of the mesophyll. Plant Cell Environ 28:1179–1190
Felton GW, Tumlinson JH (2008) Plant-insect dialogs: complex interactions at the plant-insect interface. Curr Opin Plant Biol 11:457–463
Hartley SE, DeGabriel JL (2016) The ecology of herbivore-induced silicon defences in grasses. Funct Ecol 30:1311–1322
Hewitt JE, Thrush SF, Dayton PK, Bonsdorff E (2007) The effect of spatial and temporal heterogeneity on the design and analysis of empirical studies of scale-dependent systems. Am Nat 169:398–408
Hilu KW (1984) Leaf epidermis of Andropogon sect. Leptopogon (Poaceae) in North America. Syst Bot 9:247–257
Ivey CT, De Silva N (2001) A test of the function of drip tips. Biotropica 33:188–191
Karban R (2011) The ecology and evolution of induced resistance against herbivores. Funct Ecol 25:339–347
Karban R (2015) Plant sensing and communication. Univ. Chicago Press, Chicago
Karban R, Yang LH, Edwards KF (2014) Volatile communication between plants that affects herbivory: a meta-analysis. Ecol Lett 17:44–52
Kato T, Ishida K, Kikuchi J, Torii H (2017) Induced response to herbivory in stinging hair traits of Japanese nettle (Urtica thunbergiana) seedlings in two subpopulations with different browsing pressures by sika deer. Plant Species Biol 32:340–347
Levin SA (1992) The problem of pattern and scale in ecology. Ecology 73:1943–1967
Louda SM, Rodman JE (1996) Insect herbivory as a major factor in the shade distribution of a native crucifer (Cardamine cordifolia A. Gray, bittercress). J Ecol 84:229–237
Marquis RJ (1992a) The selective impact of herbivores. In: Fritz RS, Simms EL (eds) Plant resistance to herbivores and pathogens. Chicago University Press, Chicago, pp 301–325
Marquis RJ (1992b) A bite is a bite is a bite? Constraints on response to folivory in Piper arieianum (Piperaceae). Ecology 73:143–152
Mauricio R, Bowers MD, Bazzaz FA (1993) Pattern of leaf damage affects fitness of the annual plant Raphanus sativus (Brassicaceae). Ecology 74:2066–2071
McNaughton SJ (1979) Grazing as an optimization process: grass ungulate relationships in the Serengeti. Am Nat 113:691–703
McNaughton SJ, Tarrants JL (1983) Grass leaf silicification: Natural selection for an inducible defense against herbivores. Proc Natl Acad Sci 80:790–791
Meng F, Cao R, Yang D, Niklas KJ, Sun S (2014) Trade-offs between light interception and leaf water shedding: a comparison of shade- and sun-adapted species in a subtropical rainforest. Oecologia 174:13–22
Metcalfe CR (1960) Anatomy of the Monocotyledons. I. Gramineae. Oxford Univ. Press, Oxford
Meyer GA (1998) Pattern of defoliation and its effect on photosynthesis and growth of goldenrod. Funct Ecol 12:270–279
Moore BD, Johnson SN (2017) Get tough, get toxic, or get a bodyguard: identifying candidate traits conferring belowground resistance to herbivores in grasses. Front Plant Sci 7:1925
Orrock JL, Sih A, Ferrari M, Karban R, Preisser EL, Sheriff M, Thaler J (2015) Error management in plant allocation to herbivore defense. Trends Ecol Evol 30:441–445
Ramadan A, Muroi A, Arimura G (2011) Herbivore-induced maize volatiles serve as priming cues for resistance against post-attack by the specialist armyworm Mythimna separata. J Plant Interact 6:155–158
Rubin IN, Ellner SP, Kessler A, Morrell KA (2015) Informed herbivore movement and interplant communication determine the effects of induced resistance in an individual-based model. J Anim Ecol 84:1273–1285
Sakata Y, Craig TP, Itami JK, Yamasaki M, Ohgushi T (2017) Parallel environmental factors drive variation in insect density and plant resistance in the native and invaded ranges. Ecology 98:2873–2884
Stuefer JF, Gomez S, van Molken T (2004) Clonal integration beyond resource sharing: implications for defence signaling and disease transmission in clonal plant networks. Evol Ecol 18:647–667
van der Meijden E, Wijn M, Verkaar HJ (1988) Defense and regrowth, alternative plant strategies in the struggle against herbivores. Oikos 51:355–363
Vermeij GJ (2015) Plants that lead: do some surface features direct enemy traffic on leaves and stems? Biol J Lin Soc 116:288–294
Vicari M, Bazely DR (1993) Do grasses fight back? The case for antiherbivore defences. Trends Ecol Evol 8:137–141
Wheeler AG, Krimmel BA (2015) Mirid (Hemiptera: Heteroptera) specialists of sticky plants: adaptations, interactions, and ecological implications. Annu Rev Entomol 60:393–414
Whittaker RH (1975) Communities and ecosystems, 2nd edn. Macmillan, New York
Young TP (1987) Increased thorn length in Acacia drepanolobium: an induced response to browsing. Oecologia 71:436–438
Acknowledgements
The grasses were graciously identified by Atsushi Kawakita, grasshoppers were identified by Kazuo Yamazaki, and caterpillars were identified by Koya Hashimoto and Moria Robinson. We thank Kaori Shiojiri, Kyo-hei Fukuda, Rika Ozawa, Takayuki Ohgushi, Eric LoPresti, Mikaela Huntzinger, Neal Williams, and Geerat Vermeij for discussions and technical help that improved this study. Mikaela Huntzinger read and improved the manuscript. We were supported by a grant from the Advanced Future Studies Program, Yukawa Institute of Kyoto University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Rupesh Kariyat.
Rights and permissions
About this article
Cite this article
Karban, R., Takabayashi, J. Chewing and other cues induce grass spines that protect meristems. Arthropod-Plant Interactions 13, 541–550 (2019). https://doi.org/10.1007/s11829-018-9666-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-018-9666-1