Abstract
A major problem associated with agricultural intensification over recent decades has been the development of insecticide resistance in crop pest populations. This has been a particular issue for control of the pollen beetle (Brassicogethes aeneus syn. Meligethes aeneus), a major pest of oilseed rape throughout Europe. Sustained and often prophylactic use of pyrethroid insecticides has led to the development of insecticide-resistant beetle populations, and alternatively, more environmentally benign integrated pest management strategies are sought for the pest. The population dynamics of pollen beetles and their natural enemies, and the damage caused by the pest, are influenced by processes acting at multiple scales, from the regional or landscape scale down to the local field or within-field scale. In this review, we focus on the within-field scale, and how crop management factors, including tillage, crop plant density, crop nutrition and crop rotations may be optimised and incorporated into integrated pest management strategies for more sustainable and effective control of the pest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The reliance of modern agriculture on intensive use of agrochemical inputs to maintain crop yields, along with habitat loss and simplification of crop rotations, has resulted in environmental degradation and a loss of biodiversity from arable landscapes (Bianchi et al. 2006; Robinson and Sutherland 2002; Stoate et al. 2001). This has led to a reduction in ecosystem services vital for sustainable food production, including pollination and natural pest control (Bianchi et al. 2006; Vanbergen 2013). Another major problem associated with agricultural intensification over recent decades has been the development of pesticide resistance in pathogen, pest and weed populations, in response to selection pressures arising from sustained pesticide use (Heckel 2012; Busi et al. 2013; Hahn 2014). This, coupled with declining availability of new pesticide active ingredients (due to both decreasing discovery rates and tighter legislation surrounding product approval) (Jensen 2015), represents a real threat to the efficacy of pesticide use, and consequently, to the productivity of pesticide-reliant agricultural systems. Hence, in response to these issues, there is an urgent need to develop more sustainable Integrated Pest Management (IPM) strategies for crop protection.
While the concept of IPM was initially developed by entomologists in response to specific pest-related issues, IPM (as defined within the EU under EU Framework Directive 2009/128/EC) now applies to all aspects of plant protection, and involves eight major principles (Barzman et al. 2015). Primarily, the focus of IPM is on the prevention and suppression of harmful organisms by cultural or non-pesticide means (1). Monitoring (2) followed by threshold-based decision making (3) also play a major role. Where control is necessary, non-chemical methods are prioritised over chemical pesticides (4), but where pesticides are essential to provide adequate control, the least damaging products to the environment, non-target organisms and human health are selected (5). Where pesticides are the only option, their use is kept to a minimum (6) and anti-resistance strategies (e.g. combining use of products with differing modes of action) are employed (7). The final principle involves evaluation of the control measures taken (8) (Barzman et al. 2015).
Although IPM strategies may involve consideration of landscape-scale processes (e.g. landscape-scale habitat management to manipulate pest and natural enemy populations; Skellern and Cook in press), appropriate crop management, encompassing factors such as crop rotation, cultivar choice, tillage and fertiliser regime, underpins the implementation of IPM strategies, particularly in terms of the primary principle (1) above. For example, crop rotation (i.e. the spatial and temporal diversification of cropping sequences) has been used for thousands of years as a primary means of suppressing harmful organisms by disrupting life cycle continuity of pests and pathogens associated with different crop species (Bullock 1992; Barzman et al. 2015). European maize-based cropping systems provide an illustration of this; replacement of continuous maize cultivation by incorporation of non-maize crops into the rotation has allowed for the successful management of the Western corn rootworm (Diabrotica virgifera subsp. virgifera), a pest which requires two maize cultivation cycles to complete egg-to-adult development (Vasileiadis et al. 2011).
In Europe, IPM strategies are urgently needed for control of the pollen beetle Brassicogethes aeneus F. (syn. Meligethes aeneus) (Coleoptera: Nitidulidae), a key pest of oilseed rape Brassica napus L. (OSR), a crop widely grown for cooking oil and biofuel use (EU production 19.8 M tonnes in 2016; Eurostat 2017); in this case, beetle resistance to pyrethroid insecticides, which are often applied prophylactically, has become a major problem (Zimmer et al. 2014; Thieme et al. 2010). Adult beetles emerge in early spring from overwintering habitats in woodlands and grassy areas (Rusch et al. 2012; Blazejewska 1958; Müller 1941), and feed on the flowers of many different plants before seeking OSR crops and other brassicas at the green bud growth stage, for further feeding and oviposition (Free and Williams 1978; Ouvrard et al. 2016). Feeding damage causes OSR flower bud abscission, leading to often extensive yield losses (Zlof 2008) that can approach 80% (Hansen 2004). After eggs are laid in the buds, the developing larvae feed for c. 2 weeks before dropping to the soil to pupate. New generation adults emerge in summer and again feed on pollen from plants of several families before overwintering (Williams 2010; Alford et al. 2003; Ouvrard et al. 2016). The beetles are attacked at the larval stage by a range of parasitoid species, the most abundant and widespread of which in Europe are Tersilochus heterocerus Thomson, Phradis interstitialis Thomson and Phradis morionellus Holmgren (Ulber et al. 2010b); surface-active and plant-climbing natural enemies include ground beetles and spiders (Williams et al. 2010; Frank et al. 2010).
Natural enemy regulation of pollen beetles can be substantial (Buchi 2002; Hokkanen 2008), and the possibility to manipulate both pest and natural enemy populations for beetle control, through local and landscape-scale habitat management, has received significant research interest (e.g. Cook et al. 2007; Schneider et al. 2015; Beduschi et al. 2015; Skellern and Cook in press). At the within-field scale, however, appropriate crop management also provides opportunities to improve beetle control, as crop management factors may affect plant growth and phenology (Valantin-Morison et al. 2007), which in turn influence resource quality. As specific plant quality characteristics, for example relating to bud size or glucosinolate content, are known to play a role in pollen beetle host plant selection (Nilsson 1994; Cook et al. 2006; Valantin-Morison et al. 2007; Hervé et al. 2014a), resource quality-mediated crop management effects are likely to influence pollen beetle abundance and damage in the field. Besides these effects, crop management is also likely to influence insect populations through microclimatic effects, and through variations in plant architecture, which may alter oviposition behaviour and food-seeking efficiency (Valantin-Morison et al. 2007).
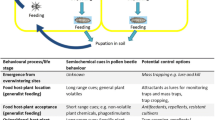
Crop management interventions to facilitate improved pollen beetle control are possible at several points within the growing cycle of OSR (summarised in Fig. 1). The crop is usually grown in rotation with cereals, at the crop planning stages, the frequency of its appearance within the rotation and cultivar choice, are important considerations. Winter OSR crops are generally sown between mid-August and mid-September (or early September in more northerly latitudes), with the exact timing of drilling often influenced by the harvesting date of the preceding (usually cereal) crop, while spring crops are drilled in March or April, usually as soon as soil conditions allow (HGCA 2014). Standard plough-based systems (e.g. ploughing followed by a power harrow-drill combination) are frequently used for OSR establishment, but reduced cultivation techniques (tine, disc or subsoiler based), and direct drilling or broadcasting into the stubble of the preceding crop are also common, as farmers look to reduce establishment costs and manage soils more sustainably (Ingram 2010; Jacobsen and Ørum 2009; Townsend et al. 2016). Target plant densities are usually in the range 25–35 plants/m2 for winter crops (HGCA 2014).
Weed control in winter OSR is achieved through pre- and/or post-emergence herbicides in the autumn, and usually further herbicide applications are necessary in the spring (HGCA 2014). In early summer, just prior to harvest, an herbicide (usually glyphosate) is frequently applied as a desiccant to aid ripening, ease harvesting and control perennial weeds for the following crop. Generally, where plough-based establishment has been used, fewer herbicides are needed (Nilsson et al. 2015). Similarly, a combination of seed treatment, and autumn and spring fungicide applications are normally required against diseases such as phoma stem canker (blackleg; Leptosphaeria maculans), sclerotinia stem rot (S. sclerotiorum), light leaf spot (Pyrenopeziza brassicae) and verticillium wilt (V. longisporum) (HGCA 2014). Plant growth regulators (some of which, including metconazole and tebuconazole, also have fungicidal action) may also be used in the spring to manipulate canopy development and reduce lodging (HGCA 2014).
In addition to base fertiliser applications made to maintain longer term soil phosphate, potassium and magnesium levels, OSR crops typically receive 150–230 kg nitrogen, and 30–60 kg sulphur ha−1 (Christen et al. 1999) in order to optimise crop growth and maximise yields. Fertilisers are usually applied in granular or liquid form using either a spinning disc applicator for granules or a crop sprayer fitted with dribble bar-type nozzles for liquids. Applications are usually split between one in the autumn and 2–3 applications in spring, ideally made between the early green and yellow bud growth stages (HGCA 2014). Typical insecticide applications to OSR crops include sprays in the autumn against cabbage stem flea beetles (Psylliodes chrysocephala) and the peach-potato aphid (Myzus persicae). Further insecticides are used at the green–yellow bud stages against pollen beetles, and during flowering, against seed weevils (Ceutorhynchus obstrictus) and pod midge (Dasineura brassicae).
While the influence of local and landscape-scale habitat management on pollen beetle abundance, damage and biocontrol are reviewed in another publication (Skellern and Cook in press), in this paper, we review crop management influences on the pest and its natural enemies, and consider how crop management may be optimised in IPM strategies for sustainable pollen beetle control.
Crop rotation
A trend towards the simplification of crop rotations over the past half-century, to include just a few crop species grown in short succession, is generally considered to have led to increases in pest proliferation and a reduction in the biodiversity of beneficial insects (Rusch et al. 2010). However, for some beneficial insects, particularly carabid beetles, little influence of rotational crop diversity changes has been seen (Holland et al. 1996; Kromp 1999). The cumulative effects of rotation-related decisions made by individual farmers are likely to exert the greatest influence on pollen beetle (and natural enemy) abundance through landscape-scale processes, as differences in the frequency of a particular crop within a rotation lead to corresponding changes in landscape areal crop proportions (Thies et al. 2008; Vinatier et al. 2012, 2013). While over a period of > 3–4 years, the introduction of oilseed rape into landscapes where the crop has not previously been grown has led to proliferation of pollen beetles as a pest (Hokkanen 2000), over the shorter term, temporally (inter-annual) and spatially increasing OSR proportions within the landscape can to lead to dilution effects on pollen beetle abundance (Zaller et al. 2008a, b; Moser et al. 2009; Schneider et al. 2015). This observation has inspired proposals for crop rotation management at the landscape scale (Beduschi et al. 2015; Schneider et al. 2015) where areas of OSR within an entire region would be progressively increased over a number of years to maintain these dilution effects, before a ‘reset’ year when little or no OSR would be grown. However, this would require a considerable amount of grower cooperation as the intervention occurs at a scale greater than the farm unit.
Cultivar
There are no commercially available cultivars of oilseed rape currently available that are marketed as resistant or tolerant to any insect pest of the crop. Although breeding for OSR resistance to pollen beetle damage has thus far received little attention, different cultivars vary in their suitability as host plants, and in their tolerance of attack. Hence, potential clearly exists to develop more resistant varieties (for a review, see Hervé and Cortesero 2016). Plant characteristics such as growth stage profile (Kruger and Ulber 2010), glucosinolate content (Cook et al. 2006; Rusch and Valantin-Morison 2013), petal colour and UV reflectance (Cook et al. 2013b), and plant size can all influence infestation and damage, and vary according to cultivar. The optimum pod number for maximum OSR yield is in the region of 6000–8000 pods/m2(Berry and Spink 2006). However, significantly more flowers than necessary for optimum pod number are usually produced, and the presence of these ‘excess flowers’ enables the crop to tolerate some pollen beetle-related flower loss before yields are reduced (Ellis and Berry 2012); excess flower numbers have been shown to vary according to cultivar (Ellis and Berry 2012; Carruthers et al. 2017) and thus are likely to be important determinant of a particular cultivar’s tolerance to pollen beetle attack. A recent study showed that, in general, cultivars bred using the cytoplasmic male-sterility (CMS) technique generally had more flowers than hybrids produced by genetic male-sterility (GMS) techniques and open-pollinated varieties (Carruthers et al. 2017). This suggests that CMS cultivars may be more tolerant to pollen beetle, although this has not been tested. Hervé et al. (2014a) identified compounds from the bud perianth, including sucrose, proline and serine that appear to act as key feeding stimulants for pollen beetles; perianth content of these compounds varied among genotypes leading to the suggestion that OSR cultivars with reduced perianth content of these compounds, in particular sucrose, could be selected to reduce damage by the pest. Furthermore, the amount of food eaten by female pollen beetles was positively related to egg numbers and size (Hervé et al. 2014b), suggesting that fewer and/or smaller eggs would also be laid on these cultivars. In another study, fewer eggs were laid on a male-sterile OSR cultivar compared with a male-fertile companion in the varietal association Synergy; larval survival was reduced and developmental time longer on the male-sterile plants (Cook et al. 2004). Manipulation of plant quality to increase larval development time has been suggested as a method to enhance biological control (Hervé et al. 2016); slower larval development on such cultivars would lengthen their window of vulnerability to parasitoid attack.
At present, no comprehensive scheme for rating oilseed rape varieties in terms of their pollen beetle resistance exists, and the current UK spray thresholds err on the side of caution and are based on the least tolerant varieties, i.e. those with few excess flowers (Ellis and Berry 2012). Potential thus clearly exists to provide farmers with information on the pollen beetle resistance characteristics of available varieties, and also reduce pest thresholds further for those with higher resistance. Cultivar choice could be an important factor to maximize the success of trap cropping strategies. Trap crops are plant stands that are grown to attract insects in order to protect target crops from attack (e.g. Hokkanen 1991). Trap cropping strategies targeted against pollen beetles in OSR has been reviewed by Mauchline et al. (2017b) and Skellern & Cook (in press). Late-flowering OSR cultivars could be used to extend the necessary growth stage differential between the main crop and an early-flowering trap crop. There is also potential for development of an OSR cultivar highly attractive to pollen beetles to function as a trap crop. Currently the best performing trap crop is turnip rape (Brassica rapa), however if early-flowering cultivars of OSR could be developed possessing the attractive properties of turnip rape, the strategy is more likely to be accepted by farmers.
Sowing date and growth stage profile
Variation in sowing date will influence some pests through alteration of the synchrony between lifecycles and susceptible crop growth stages. For OSR, the difference between winter and spring sowing has the greatest effect, and because the damage-susceptible stages of the crop occur at peak pest abundance, spring sown crops in particular are more susceptible to flea beetle (Phyllotreta spp.) and pollen beetle damage (Alford et al. 2003; Dodsall and Stephenson 2005). For the latter, however, as excess flower numbers do not differ between winter and spring crops (Ellis and Berry 2012), it is likely that pollen beetle spillover from large areas of winter crops onto smaller areas of spring crops leads to locally increased pest pressure through beetle concentration effects (except for in more northerly regions where spring crops predominate). For winter crops, an early study found that later sowing dates limited pollen beetle damage (Vasak 1983), and it is possible that this was related to reduced fecundity of older beetles on the less advanced crops. Results from another study have shown that the fecundity of beetle populations maintained on Raphanus sativus late into the season was indeed low (Skellern et al. in prep). By contrast, Rusch et al. (2013b) found that sowing date was not an important predictor of pollen beetle abundance or plant damage, and while earlier sowing can increase root fly damage but decrease cabbage stem flea beetle attack, Valantin-Morison et al. (2007) also found little effect of sowing date on pollen beetle damage.
Pollen beetles often feed on early spring-flowering plants before they move onto oilseed rape (Free and Williams 1978; Ouvrard et al. 2016), and have been observed on the crop at pre-green bud growth stages (Veromann et al. 2012); during most seasons, it seems that the beetles arrive sufficiently early to colonise even the most advanced crops before the susceptible green bud stages are over (Cook et al. 2006; Mauchline et al. 2017a). Relative differences in growth stage, for example between a crop and trap crop or variation within a field, however, are very important in determining infestation levels, because of the beetle’s preference for the flowering growth stages (Cook et al. 2007; Mauchline et al. 2017a), which may provide a reliable cue for the availability of buds of the preferred size for oviposition (2–3 mm; Ekbom and Borg 1996), together with food resources (Frearson et al. 2005).
The coincidence between pollen beetle parasitoid emergence and crop development is not precise (Nilsson 1985) and there is some evidence that the parasitoids may be more sensitive to asynchrony therein than their hosts. Larvae present on early flowering winter-sown turnip rape varieties in some seasons may escape parasitisation, probably because the parasitoids do not arrive until after the bud stages are complete (Skellern et al. in prep). It is thus possible that in some seasons, larvae on very early flowering oilseed rape crops may similarly escape parasitisation.
Plant density
Oilseed rape plants have potential to compensate for pollen beetle-related floral bud losses by producing new flower buds carried by either existing or new branches (Tatchell 1983; Nilsson 1994; Williams and Free 1979). Plant density, which can be strongly influenced by crop management through seed rates and sowing date, however, impacts the ability of the crop to compensate for bud losses, with higher planting densities generally leading to more restricted branching as there is less space for compensatory growth (Leach et al. 1999; Momoh and Zhou 2001). Despite this, some studies have shown little or no influence of plant density on pollen beetle abundance or damage (Rusch et al. 2013b; Ferguson et al. 2003), while others have shown negative relationships (Hurej and Twardowski 2006; Valantin-Morison et al. 2007). It is likely that lower beetle abundance and damage at higher plant densities could occur through dilution effects. Excess flower numbers (defined as the difference between flower and final pod numbers) produced per plant are strongly negatively affected by increasing plant density, meaning that on a per plant basis, beetle susceptibility increases (hence, economic damage thresholds decrease; Ellis and Berry 2012). Although Leach et al. (1999) found fewer branches per plant at high densities, denser crops had more branches per square metre so on a per unit area basis, total excess flower numbers may increase with plant density, explaining why more densely planted crops may suffer less damage. Additionally, Valantin-Morison et al. (2007) observed that root fly (Delia radicum), cabbage stem flea beetle (Psylliodes chrysocephala) and rape stem weevil (Ceutorhynchus napi) damage all decreased with increasing plant density; this too may have been due to dilution effects, but increased competition between plants at higher densities may have reduced resource quality for these insects.
Pollen beetle parasitism rates have been shown to have a positive relationship with plant density (Zaller et al. 2009a), and it is probable that parasitoid searching efficiency is improved where racemes (and hence host larvae) are more closely spaced. Interestingly, Zaller et al. (2009a) also showed that oilseed rape 1000 kernel mass was positively related to pollen beetle parasitism rates, suggesting that the parasitoids may have influenced beetle capacity to cause damage to the growing crop, perhaps through disturbance or interference effects.
Weed management
There has been little, if any, research on the direct effects of weeds on pollen beetle infestation of OSR crops. Similarly, the direct effects of weed management on pollen beetle regulation by natural enemies has been little studied, however, generalist predators such as carabids and spiders may benefit from increased weed cover through provision of shelter and alternative sources of food/prey (Speight and Lawton 1976; Holland et al. 1999; Schmidt et al. 2005; Sunderland and Samu 2000). In particular, omnivorous carabid species that consume weed seeds as well as prey have been shown to be associated with increased weed cover (Kulkarni et al. 2017; Diehl et al. 2012). Potentially, therefore, less intensive weed management regimes may enhance pollen beetle regulation by these natural enemies. However, more work is needed in this area as positive relationships between weed density and generalist predators may not necessarily lead to better pest suppression; alternative weed-associated resources may distract generalist enemies from target pests (Diehl et al. 2012).
To our knowledge, no study has investigated the effect of weed management on pollen beetle specialist parasitoids. However, it is possible that access to weed-derived pollen and nectar could enhance the efficacy of these natural enemies, and that they may benefit from reduced weed management intensity. Floral resource utilisation has been shown to increase parasitoid longevity (Lee et al. 2004; Robinson et al. 2008) and fecundity (Hogg et al. 2011; Baggen and Gurr 1998; Winkler et al. 2006), and the pollen beetle parasitoid T. heterocerus is known to acquire nectar while foraging in the field (Rusch et al. 2013a). However, the massive floral resources supplied by the OSR crop itself may mean that the benefits of weed-derived nectar and pollen are marginal, or depend on the phenology of different parasitoid species in relation to that of the crop. For example, P. interstitialis, which is active at the OSR bud stages (i.e. before the flowers are open) may benefit more from weed-borne floral resources than P. morionellus or T. heterocerus which tend to colonise the crop only at the beginning of flowering (Ulber and Nitzsche 2006; Williams 2006). The benefits delivered by weeds to natural enemies of pollen beetles could also be realised through managed companion cropping within-crop (e.g. Howard 2016) or via sown field margins at the field boundry (Baverstock et al. 2014; Skellern & Cook in press), which avoid the negative impact of weeds. However both tactics require further research and development.
Growth regulators
Several compounds, including metconazole and tebuconazole, both of which also have fungicidal action, are used as growth regulators on OSR to manipulate canopy development, root growth and stem extension (HGCA 2014). It seems, however, that no research has been carried out on the influence of these compounds on pest abundance or damage. As plant height can positively influence both pollen beetle and stem weevil Ceutorhynchus pallidactylus infestation (Schlinkert et al. 2015, 2016; Ferguson et al. 2003), it is possible that these compounds could be used to manipulate populations of these pests. Additionally, research is needed on the use of these or similar compounds to influence the timing of bud and flowering growth stages, as potential may exist to use growth regulators as an alternative to, or to enhance trap cropping strategies.
Crop nutritional status
Several studies have revealed relationships between crop nutritional status and pollen beetle abundance on OSR. Zaller et al. (2008b) demonstrated a curvilinear relationship between pollen beetle infestation and soil index (a measure used in Austria of the natural yield capacity of soils, on a scale of 0–100, with soils of the highest yield capacity in the country scoring 100, and taking into account soil type, humus content, soil depth, texture, density, structure, lime content, gleying and soil congregation; ÖBG 2001); beetle abundance increased with soil index to average index levels, but then declined at higher values. The authors suggested that at low soil index values, plant quality may have been unsuitable for the pest, but at higher values, the crop may be better able to protect itself from herbivore attack. Indeed, plant glucosinolate content, the breakdown products of which are important for pollen beetle host plant location (Blight and Smart 1999; Cook et al. 2006), has been shown to increase with nutrient supply (Markus et al. 1996), but on higher quality soils, the production of other secondary compounds may enhance plant defences against the pest (Cipollini and Bergelson 2002). Despite these soil quality effects, Zaller et al. (2008b) observed no direct influence of variation in nitrogen fertiliser rates (range 45–143 kg ha−1), perhaps because applications may not have accurately reflected soil nitrogen availability. By contrast, Culjak et al. (2011) found greater pollen beetle abundance on plants treated with lower and higher N rates (of 69 and 115 kg ha−1 respectively) than on those treated at medium rates (92 kg ha−1), and Veromann et al. (2013) were also able to demonstrate an influence of nitrogen fertiliser, with increased beetle infestations occurring on both low (60 and 80 kg ha−1 N) and high (160 kg ha−1) rate nitrogen-treated plots, compared with zero and medium (100, 120 and 140 kg ha−1) rate treatments. In this case, there was some evidence that differences in infestation rates were mediated through quantitative changes in plant volatile profiles which differed according to nitrogen application rates. In particular, emissions of acetic acid, methyl salicylate and several lipoxygenase pathway volatiles were greater from plants treated at the higher N rates.
It is possible that plant nutrition-mediated differences in visual cues may influence infestation and damage by pollen beetles. Higher nitrogen rates can increase OSR floral spectral reflectance in the UV wavelengths, and in those above 530 nm (Blake et al. 2014); visual stimuli at these wavelengths are known to elicit positive behavioural responses in pollen beetles (Döring et al. 2012; Cook et al. 2013b). Indeed, for the seed weevil, plant nitrogen and sulphur nutrition has been shown to influence attraction to OSR through differences in leaf reflectance characteristics (Blake et al. 2014). As well as altering spectral reflectance characteristics, higher nitrogen rates can lead to increased plant size and height (Grant and Bailey 1993), which can also influence pollen beetle and stem weevil abundance and damage (as discussed above).
In a study investigating both crop management and landscape influences on OSR pest populations and crop damage, Rusch et al. (2013b) reported that crop nitrogen status (as determined by calculation of the nitrogen nutrition index; Lemaire and Meynard 1997) was of little importance in determining pollen beetle infestation, but represented a very important predictor of crop damage; high nitrogen status plants exhibited low proportions of damaged buds. Another study (Valantin-Morison et al. 2007) also showed a similar effect, with reduced proportions of flowers destroyed by pollen beetles on higher nitrogen status soils. In both cases, it is likely that the negative relationship between nitrogen nutrition and pollen beetle damage is related to the greater capacity of higher nutrient status plants to produce defensive secondary compounds (Cipollini and Bergelson 2002) and to compensate for damage, particularly via the production of new racemes (Podlaska et al. 1996).
There is little evidence in the literature of direct crop nutritional status effects on pollen beetle natural enemies; rather, pollen beetle parasitism rates appear to be influenced indirectly through host density-dependence effects (Zaller et al. 2009b; Veromann et al. 2013). Similarly, the activity-density of Amara similata (Carabidae) females was found to be negatively correlated with soil index (Haschek et al. 2012), and this is most likely related to prey and/or weed seed abundance; on more productive soils, readily available food resources lead to satiated beetles that are relatively inactive and less likely to be trapped (Lenski 1984).
In general, it seems that the increased capability of high nutrient status crops (particularly with regard to nitrogen) to compensate for pest damage can often offset the effect of greater pollen beetle abundance on these crops, and timing of fertiliser applications to ensure sufficient nutrient availability at the susceptible green to yellow bud growth stages may serve to curb yield losses. Indeed, in dry seasons when nitrogen uptake is limited, it is possible that crops would benefit from foliar nitrogen applications during the damage-susceptible stages.
Insecticide regimes
The insecticides that are routinely used to target pollen beetles and other OSR pests have detrimental effects on natural enemy populations and parasitism rates (Hanson et al. 2015; Nilsson et al. 2015; Jansen and San Martin 2014), meaning that integration of chemical and conservation biological control into IPM strategies is a major challenge (Williams 2004, 2006). These undesirable side effects, however, may be ameliorated in a several ways. Choice of lower toxicity products such as tau-fluvalinate over other pyrethroids (Klukowski 2006) may enhance parasitoid survival. Jansen and San Martin (2014) found that pymetrozine had limited effects on parasitoid mortality compared with thiacloprid, phosmet, chlorpyriphos-ethyl and tau-fluvalinate. Pymetrozine, tau-fluvalinate and phosmet did not affect the ratio of parasitoids: pollen beetles at the end of the season while thiacloprid and chlorpyriphos-ethyl were found to alter this ratio in the pollen beetles’ favour. Reduction of insecticide applications is possible through the use of decision support systems and better use of pest thresholds (Johnen et al. 2010; Ferguson et al. 2016; Junk et al. 2016; Ferguson and Cook 2014). Indeed, in the future, potential for the development of combined thresholds based on multiple pest species, rather than on each pest individually may serve to further reduce insecticide use, as a recent study has shown, counterintuitively, that the interactive effects of high levels of plant damage by seed and stem weevils can lead to increased OSR seed yield, presumably due to plant overcompensation effects (Gagic et al. 2016). Recent research into using more benign natural products, particularly stone meal, Silico-sec (inert silica diatomaceous earth) and liquid manure as alternatives to insecticides have shown promising results in terms of pollen beetle control (Dorn et al. 2014), but further research is needed into effects on non-target insects, timing and frequency of applications and the persistence of formulations. Better targeting of insecticide applications relative to pest incidence and parasitoid phenology also has potential to lower parasitoid mortality. The main activity period of pollen beetle parasitoids extends from the late bud stages to beyond the end of flowering, and consequently, insecticides applied during flowering (typically targeting seed weevils and pod midge) have particularly deleterious effects (Ulber et al. 2010a). Thus, avoidance of insecticide applications during the flowering period and restriction of those targeting pollen beetles to the earlier green bud stages could reduce negative effects on natural pest control. The time of day at which insecticides are applied could also influence the survival of beneficial insects. Ferguson et al. (2013) found that during flowering, the peak flight activity of both pollen beetles and the parasitoid Phradis interstitialis was around midday, and few insects were caught before 10:00 am. Further research, however, is needed to determine whether insecticide applications early or late in the day could maintain pollen beetle control yet reduce parasitoid mortality.
Spatial targeting of insecticides only to areas of high pest density is another means by which parasitoid mortality could be reduced. Ferguson et al. (2003) noted that while P. interstitialis had a close spatial association with its host, another parasitoid, Tersilochus heterocerus was more evenly distributed across the field than the pollen beetle larvae, thus insecticide targeting to only the beetle-dense areas could help to conserve T. heterocerus at least. Where a trap crop is used, the application of insecticide to the trap crop before the beetles move on to the main crop also has the potential to improve spatial targeting (Cook et al. 2013a; Čuljak et al. 2016). It is important to note, however, that where insecticides are used, even if direct effects on beneficial insects are reduced by these strategies, their populations or activity are still likely to be reduced, possibly by sub-lethal effects, or simply through the reduction of available hosts. Unsprayed field margin areas where brassicas are present are therefore likely to be of great importance in maintaining populations of brassica-specialist pollen beetle parasitoids (Skellern et al. in prep).
Tillage
The varying degree of disturbance caused by different pre- and post-OSR soil tillage regimes can differentially impact abundance and survival of pollen beetle parasitoids and generalist predators. In particular, because pollen beetle parasitoids overwinter in the soil of former oilseed rape fields (Nitzsche and Ulber 1998), leaving fallow ground or using reduced-disturbance forms of tillage such as direct drilling in the establishment of the crops following oilseed rape can greatly enhance parasitoid survival compared with plough-based systems. Experiments in Sweden and Finland showed that overwintering survival of parasitoids was around four times higher from fallow or direct drilling treatments, compared with ploughing or disc-based non-inversion techniques (Nilsson 1985; Hokkanen et al. 1988). Ferguson et al. (2007) gave similar results with fallow treatments showing the highest survival rates, followed by non-inversion, and plough-based treatments the lowest. Other studies from Germany have also confirmed the detrimental effects of ploughing on parasitoid survival (Nitzsche and Ulber 1998; Wahmhoff et al. 1999). Hanson et al. (2015), however, detected no difference in parasitoid emergence following ploughing vs disc- or tine-based tillage, but made no comparisons with post-OSR fallow or direct drilling techniques.
There is some evidence that generalist predators, particularly spiders, are influenced by tillage regime with ploughing showing more disruptive effects than lower disturbance methods (House and Stinner 1983; Haskins and Shaddy 1986). However, other studies have shown a beneficial effect of pre-OSR ploughing on Erigone and Oedothorax (Linyphiidae) populations, but no influence of post-OSR tillage regime (Williams and Ferguson 2008). The same study showed no impact of pre- or post-OSR tillage on carabid numbers or species richness. Many carabid species overwinter in field boundary habitats and migrate into crops in spring and summer and thus may avoid autumn tillage-related injury, but if ploughing is used in the establishment of spring crops, more detrimental effects are seen (Büchs 2003).
Although pollen beetles and their parasitism rates can be indirectly influenced (via growth stage and density-dependence effects) by the tillage treatments used in OSR crop establishment (Williams & Ferguson, 2008), the tillage methods used post-OSR have little effect on the pest, as unlike their parasitoids, the adults overwinter elsewhere and not within-field (Rusch et al. 2012). This implies that the encouragement of low-disturbance tillage methods for the establishment of crops following OSR, in particular direct drilling, presents an opportunity to enhance parasitoid populations without having detrimental effects on generalist predators, and without having positive effects on the pest.
Comparisons of crop management strategies for OSR
An integrated crop management strategy (ICM) for OSR, based on reduced tillage (to enhance parasitoid survival, and to reduce crop establishment-related energy use) and spraying to pest thresholds has been compared with ‘standard’ agronomic procedures (plough-based tillage and spraying to schedule) as part of an EU project (Nilsson et al. 2015). Standard and ICM procedures were compared at sites in Germany, Poland, Sweden and the UK, in terms of their effects on pests, yields, energy use and crop production costs. Insecticide applications were more than halved where control thresholds were used, and parasitism rates in aggregate across three major pests of OSR (seed and stem weevils, and pollen beetles) were significantly higher in untreated than sprayed plots. While the ICM system generally needed an extra herbicide treatment, because of the need to curb weeds otherwise controlled through ploughing, and yields were slightly lower, total production costs and energy use were also lower for the ICM system, meaning that growing the OSR under ICM would often be economically favourable for farmers, and more environmentally sustainable. These results are encouraging, but potential clearly exists to improve upon the ICM system studied, by integrating more of the crop management practices/aspects discussed above, favouring better control of pollen beetles and other OSR pests.
Conclusions
Crop management has important influences on pollen beetle abundance, the damage caused and on natural enemy populations and activity. It seems that more dense, high nutrient status crops (particularly with regard to nitrogen availability at damage-susceptible growth stages) are the most tolerant of attack and these conditions also facilitate parasitoid activity through density-dependence effects and improved host-seeking efficiency. Choice of cultivar is also important, but in the absence of a standard pollen beetle resistance rating scheme, farmers have little basis on which to make this decision. Scope exists to adapt the spatial and temporal targeting of insecticides, or to use more benign products to reduce detrimental effects on non-target beneficial insects. The potential to use growth regulators to manipulate pest populations warrants further investigation, and reduced tillage establishment techniques following the OSR crop should be encouraged to enhance parasitoid survival between seasons. Crop rotation can have important effects on pest abundance, but these are mediated mostly at the landscape scale. Indeed, the implications of crop management tactics for pollen beetles and their natural enemies need to be considered alongside landscape-scale processes, as both influence insect abundance and damage. On balance, it seems that landscape-scale processes may be the more important determinant of pollen beetle abundance (Rusch et al. 2013b), while damage is a product of both landscape-scale processes and, through management, the crop’s injury-compensation capability (Rusch et al. 2013b; Valantin-Morison et al. 2007).
References
Alford DV, Nilsson C, Ulber B (2003) Insect pests of oilseed rape crops. In: Alford DV (ed) Biocontrol of oilseed rape pests. Blackwell Science, Oxford, pp 9–42
Baggen LR, Gurr GM (1998) The influence of food on Copidosoma koehleri (Hymenoptera: Encyrtidae), and the use of flowering plants as a habitat management tool to enhance biological control of potato moth, Phthorimaea operculella (Lepidoptera: Gelechiidae). Biol Control 11:9–17. doi:10.1006/bcon.1997.0566
Barzman M, Bàrberi P, Birch ANE, Boonekamp P, Dachbrodt-Saaydeh S, Graf B, Hommel B, Jensen JE, Kiss J, Kudsk P, Lamichhane JR, Messéan A, Moonen A-C, Ratnadass A, Ricci P, Sarah J-L, Sattin M (2015) Eight principles of integrated pest management. Agron Sustain Dev 35:1199–1215. doi:10.1007/s13593-015-0327-9
Baverstock J, Pell JK, Storkey J, Torrance MT, Cook SM (2014) Field margins for biocontrol and biodiversity across crop rotations: overview of the aims and approaches of Defra project IF01122. IOBC/wprs Bull 100:29–33
Beduschi T, Tscharntke T, Scherber C (2015) Using multi-level generalized path analysis to understand herbivore and parasitoid dynamics in changing landscapes. Landsc Ecol 30:1975–1986. doi:10.1007/s10980-015-0224-2
Berry PM, Spink JH (2006) A physiological analysis of oilseed rape yields: past and future. J Agric Sci 144:381–392. doi:10.1017/S0021859606006423
Bianchi FJ, Booij CJ, Tscharntke T (2006) Sustainable pest regulation in agricultural landscapes: a review on landscape composition, biodiversity and natural pest control. Proc R Soc Lond B 273:1715–1727. doi:10.1098/rspb.2006.3530
Blake AJ, Dosdall LM, Tansey JA (2014) Nutritional effects on the appearance of canola and its attractiveness to the cabbage seedpod weevil. J Insect Behav 27:759–775. doi:10.1007/s10905-014-9466-0
Blazejewska A (1958) Zimowanie slodyszka rzepakowego (Meligethes aeneus Fabr.). Stud Soc Sci Torun 4:5–30
Blight MM, Smart LE (1999) Influence of visual cues and isothiocyanate lures on capture of the pollen beetle, Meligethes aeneus in field traps. J Chem Ecol 25:1501–1516
Buchi R (2002) Mortality of pollen beetle (Meligethes spp.) larvae due to predators and parasitoids in rape fields and the effect of conservation strips. Agric Ecosyst Environ 90:255–263. doi:10.1016/s0167-8809(01)00213-4
Büchs W (2003) Predators as biocontrol agents of oilseed rape pests. In: Alford DV (ed) Biocontrol of oilseed rape pests. Blackwell Scientific, Oxford, pp 279–298
Bullock DG (1992) Crop rotation. Crit Rev Plant Sci 11:309–326. doi:10.1080/07352689209382349
Busi R, Vila-Aiub MM, Beckie HJ, Gaines TA, Goggin DE, Kaundun SS, Lacoste M, Neve P, Nissen SJ, Norsworthy JK (2013) Herbicide-resistant weeds: from research and knowledge to future needs. Evol Appl 6:1218–1221
Carruthers JM, Cook SM, Wright GA, Osborne JL, Clark SJ, Swain JL, Haughton AJ (2017) Oilseed rape (Brassica napus) as a resource for farmland insect pollinators: quantifying floral traits in conventional varieties and breeding systems. GCB Bioenergy. doi:10.1111/gcbb.12438
Christen O, Evans E, Nielsson C, Haldrup C (1999) Oilseed rape cropping systems in NW Europe. In: Proceedings of the 10th International Rapeseed Congress, 26–29 September 1999, Canberra, Australia. http://www.regional.org.au/au/gcirc/2/96.htm. Accessed January 2017
Cipollini DF, Bergelson J (2002) Interspecific competition affects growth and herbivore damage of Brassica napus in the field. Plant Ecol 162:227–231. doi:10.1023/a:1020377627529
Cook SM, Murray DA, Williams IH (2004) Do pollen beetles need pollen? The effect of pollen on oviposition, survival and development of a flower-feeding herbivore. Ecol Entomol 29:164–173
Cook SM, Smart LE, Martin JL, Murray DA, Watts NP, Williams IH (2006) Exploitation of host plant preferences in pest management strategies for oilseed rape (Brassica napus). Entomol Exp Appl 119:221–229. doi:10.1111/j.1570-7458.2006.00419.x
Cook SM, Rasmussen HB, Birkett MA, Murray DA, Pye BJ, Watts NP, Williams IH (2007) Behavioural and chemical ecology underlying the success of turnip rape (Brassica rapa) trap crops in protecting oilseed rape (Brassica napus) from the pollen beetle (Meligethes aeneus). Arthropod Plant Interact 1:57–67. doi:10.1007/s11829-007-9004-5
Cook SM, Doring TF, Ferguson AW, Martin JL, Skellern MP, Smart LE, Watts NP, Welham SJ, Woodcock C, Pickett JA (2013a) Development of an integrated pest management strategy for control of pollen beetles in winter oilseed rape. HGCA project report no. 504. http://www.hgca.com/media/198124/pr504.pdf. Accessed January 2017
Cook SM, Skellern MP, Döring TF, Pickett JA (2013b) Red oilseed rape? The potential for manipulation of petal colour in control strategies for the pollen beetle (Meligethes aeneus). Arthropod Plant Interact 7:249–258. doi:10.1007/s11829-013-9252-5
Culjak TG, Grubisic D, Juran I (2011) The effect of fertilisers on the pests of oilseed rape. J Food Agric Environ 9:428–430
Čuljak TG, Pernar R, Juran I, Ančić M, Bažok R (2016) Impact of oilseed rape crop management systems on the spatial distribution of Brassicogethes aeneus (Fabricius 1775): implications for integrated pest management. Crop Prot 89:129–138
Diehl E, Wolters V, Birkhofer K (2012) Arable weeds in organically managed wheat fields foster carabid beetles by resource- and structure-mediated effects. Arthropod Plant Interact 6:75–82. doi:10.1007/s11829-011-9153-4
Dodsall LM, Stephenson FC (2005) Managing flea beetles (Phyllotreta spp.) (Colepotera: Chrysomelidae) in canola with seeding date, plant density and seed treatment. Agron J 97:1570–1578
Döring TF, Skellern MP, Watts NP, Cook SM (2012) Colour choice behaviour in the pollen beetle, Meligethes aeneus (Coleoptera: Nitidulidae). Physiol Entomol 37:360–378
Dorn B, Jossi W, Humphrys C, Hiltbrunner J (2014) Screening of natural products in the laboratory and the field for control of pollen beetles. J Appl Entomol 138:109–119. doi:10.1111/jen.12086
Ekbom B, Borg A (1996) Pollen beetle (Meligethes aeneus) oviposition and feeding preference on different host plant species. Entomol Exp Appl 78:291–299
Ellis S, Berry P (2012) Re-evaluating thresholds for pollen beetle in oilseed rape. HGCA publication PR495. https://cereals.ahdb.org.uk/media/200518/pr495.pdf. Accessed January 2017
Eurostat (2017) Agriculture database. http://ec.europa.eu/eurostat/web/agriculture/data/database. Accessed January 2017
Ferguson AW, Cook SM (2014) Results of a small survey amongst farmers and advisers in the UK on their evaluation of the proPlant pollen beetle migration tool and its influence on their practice. IOBC/WPRS Bull 104:97–104
Ferguson AW, Klukowski Z, Walczak B, Clark SJ, Mugglestone MA, Perry JN, Williams IH (2003) Spatial distribution of insect pests in oilseed rape: implications for integrated pest management. Agric Ecosyst Environ 95:509–521
Ferguson AW, Holdgate R, Mason NS, Williams IH (2007) Non-inversion tillage to conserve functional biodiversity for biocontrol of oilseed rape pests. Proceedings of the XVI International Plant Protection Congress, 15–18 October 2007, Glasgow, UK:818–819
Ferguson AW, Holdgate R, Mason NS, Clark SJ, Williams IH (2013) Phenologies and dial periodicities of within-crop flight by pests and parasitoids in winter oilseed rape in the UK. IOBC/WPRS Bull 92:45–54
Ferguson AW, Skellern MP, Johnen A, von Richthofen J-S, Watts NP, Bardsley E, Murray DA, Cook SM (2016) The potential of decision support systems to improve risk assessment for pollen beetle management in winter oilseed rape. Pest Manag Sci 72:609–617. doi:10.1002/ps.4069
Frank T, Drapela T, Moser D, Zaller JG (2010) Insect pests and spiders in oilseed rape and their response to site and landscape factors. In: Williams IH (ed) Biocontrol-based integrated management of oilseed rape pests. Springer, London, pp 285–304
Frearson DJT, Ferguson AW, Campbell JM, Williams IH (2005) The spatial dynamics of pollen beetles in relation to inflourescence growth stage of oilseed rape: implications for trap crop strategies. Entomol Exp Appl 116:21–29
Free JB, Williams IH (1978) Responses of the pollen beetle Meligethes aeneus, and the seed weevil Ceuthorhynchus assimilis, to oilseed rape Brassica napus, and other plants. J Appl Ecol 15:761–774
Gagic V, Riggi LG, Ekbom B, Malsher G, Rusch A, Bommarco R (2016) Interactive effects of pests increase seed yield. Ecol Evol 6:2149–2157
Grant C, Bailey L (1993) Fertility management in canola production. Can J Plant Sci 73:651–670
Hahn M (2014) The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J Chem Biol 7:133–141. doi:10.1007/s12154-014-0113-1
Hansen LM (2004) Economic damage threshold model for pollen beetles (Meligethes aeneus F.) in spring oilseed rape (Brassica napus L.) crops. Crop Prot 23:43–46. doi:10.1016/S0261-2194(03)00167-4
Hanson HI, Smith HG, Hedlund K (2015) Agricultural management reduces emergence of pollen beetle parasitoids. Agric Ecosyst Environ 205:9–14. doi:10.1016/j.agee.2015.03.001
Haschek C, Drapela T, Schuller N, Fiedler K, Frank T (2012) Carabid beetle condition, reproduction and density in winter oilseed rape affected by field and landscape parameters. J Appl Entomol 136:665–674. doi:10.1111/j.1439-0418.2011.01694.x
Haskins MF, Shaddy JH (1986) The ecological effects of burning, mowing and ploughing on ground inhabiting spiders (Araneae) in an old-field ecosystem. J Arachnol 14:1–14
Heckel DG (2012) Insecticide resistance after silent spring. Science 337:1612–1614
Hervé MR, Cortesero AM (2016) Potential for oilseed rape resistance in pollen beetle control. Arthropod Plant Interact 10:463–475. doi:10.1007/s11829-016-9438-8
Hervé MR, Delourme R, Gravot A, Marnet N, Berardocco S, Cortesero AM (2014a) Manipulating feeding stimulation to protect crops against insect pests? J Chem Ecol 40:1220–1231
Hervé MR, Delourme R, Leclair M, Marnet N, Cortesero AM (2014b) How oilseed rape (Brassica napus) genotype influences pollen beetle (Meligethes aeneus) oviposition. Arthropod Plant Interact 8:383–392. doi:10.1007/s11829-014-9321-4
Hervé MR, Delourme R, Cortesero AM (2016) Plant genotype affects the quality of oilseed rape (Brassica napus) for adults and larvae of the pollen beetle (Meligethes aeneus). Physiol Entomol 41:202–209. doi:10.1111/phen.12143
HGCA (2014) HGCA Oilseed rape guide. http://www.hgca.com/media/305093/g55-oilseed-rape-guide-jan-2014-update.pdf. Accessed January 2017
Hogg BN, Nelson EH, Mills NJ, Daane KM (2011) Floral resources enhance aphid suppression by a hoverfly. Entomol Exp Appl 141:138–144. doi:10.1111/j.1570-7458.2011.01174.x
Hokkanen HMT (1991) Trap cropping in pest management. Annu Rev Entomol 36:119–138
Hokkanen H (2000) The making of a pest: recruitment of Meligethes aeneus onto oilseed Brassicas. Entomol Exp Appl 95:141–149
Hokkanen HMT (2008) Biological control methods of pest insects in oilseed rape. Bull OEPP 38:104–109
Hokkanen H, Husberg GB, Söderblom M (1988) Natural enemy conservation for the integrated control of the rape blossom beetle Meligethes aeneus F. Ann Agric Fenn 27:281–294
Holland JM, Dryslade A, Hewitt MV, Turley D (1996) The LINK IFS project—the effect of crop rotations and cropping systems on Carabidae. Asp Appl Biol 47:119–126
Holland JM, Perry JN, Winder L (1999) The within-field spatial and temporal distribution of arthropods in winter wheat. Bull Entomol Res 89:499–513
House GJ, Stinner BR (1983) Arthropods in no-tillage soybean agroecosystems: community composition and ecosystem interactions. Environ Manag 6:23–28
Howard A (2016) The potential for companion cropping and intercropping on UK arable farms. Nuffield Farming Scholarships Trust Report. http://nuffieldinternational.org/rep_pdf/1474016405Andrew-Howard-report-2015.pdf
Hurej M, Twardowski J (2006) Effects of oilseed rape plant density on herbivores. Prog Plant Prot 46:374–377
Ingram J (2010) Technical and social dimensions of farmer learning: an analysis of the emergence of reduced tillage systems in England. J Sustain Agric 34:183–201. doi:10.1080/10440040903482589
Jacobsen BH, Ørum JE (2009) Farm economic and environmental effects of reduced tillage. Acta Agric Scand Sect C Food Econ 6:134–142. doi:10.1080/16507540903549474
Jansen J-P, San Martin G (2014) A large field trial to assess the short-term and long-term effects of 5 insecticides used to control the pollen beetle on parasitic hymenoptera in oilseed rape. IOBC/WPRS Bull 103:9–16
Jensen JE (2015) Perspectives on the implementation of IPM in EU—the advisory perspective. In: Paper given at IPM innovation in Europe, Poznan, Poland January 15–17, 2015. www.pure-ipm.eu/node/430. Accessed April 2017
Johnen A, Williams IH, Nilsson C, Klukowski Z, Luik A, Ulber B (2010) The proPlant decision support system: phenological models for the major pests of oilseed rape and their key parasitoids in Europe. In: Williams IH (ed) Biocontrol-based integrated management of oilseed rape pests. Springer, London, pp 381–404
Junk J, Jonas M, Eickermann M (2016) Assessing meteorological key factors influencing crop invasion by pollen beetle (Meligethes aeneus F.)—past observations and future perspectives. Meteorol Z 25:357–364. doi:10.1127/metz/2015/0665
Klukowski Z (2006) The impact of insecticide application and timing on parasitoid activity and levels of parasitism. In: Proceedings of the international symposium on integrated pest management in oilseed rape, 3–5 April 2006, Gottingen, Germany
Kromp B (1999) Carabid beetles in sustainable agriculture: a review on pest control efficacy, cultivation impacts and enhancement. Agric Ecosyst Environ 74:187–228. doi:10.1016/s0167-8809(99)00037-7
Kruger ML, Ulber B (2010) Influence of different winter oilseed rape cultivars on the reproduction and population density of the pollen beetle, Meligethes aeneus. Julius-Kuhn-Archiv 428:257–258
Kulkarni SS, Dosdall LM, Spence JR, Willenborg CJ (2017) Field density and distribution of weeds are associated with spatial dynamics of omnivorous ground beetles (Coleoptera: Carabidae). Agric Ecosyst Environ 236:134–141. doi:10.1016/j.agee.2016.11.018
Leach JE, Stevenson HJ, Rainbow AJ, Mullen LA (1999) Effects of high plant populations on the growth and yield of winter oilseed rape (Brassica napus). J Agric Sci 132:173–180. doi:10.1017/s0021859698006091
Lee JC, Heimpel GE, Leibee GL (2004) Comparing floral nectar and aphid honeydew diets on the longevity and nutrient levels of a parasitoid wasp. Entomol Exp Appl 111:189–199. doi:10.1111/j.0013-8703.2004.00165.x
Lemaire G, Meynard J (1997) Use of the nitrogen nutrition index for the analysis of agronomical data. In: Lemaire G (ed) Diagnosis of the nitrogen status in crops. Springer, Berlin, pp 45–55
Lenski RE (1984) Food limitation and competition: a field experiment with two Carabus species. J Anim Ecol 53:203–216
Markus J, Podlaska J, Dmoch J, Pietkiewicz S, Loboda T, Lewandowski M (1996) Compensation of the damage caused by pollen beetle (Meligethes aeneus) on winter oilseed rape under different plant density and fertilisation: III. Chemical composition of winter oilseed rape cv. Leo. Rośliny Oleiste 17:325–330
Mauchline AL, Cook SM, Powell W, Chapman JW, Osborne JL (2017a) Migratory flight behaviour of the pollen beetle Meligethes aeneus. Pest Manag Sci. doi:10.1002/ps.4550
Mauchline AL, Hervé MR, Cook SM (2017b) Semiochemical based alternatives to synthetic toxicant insecticides for pollen beetle management. Arthropod Plant Interact. doi:10.1007/s11829-017-9569-6
Momoh EJJ, Zhou W (2001) Growth and yield responses to plant density and stage of transplanting in winter oilseed rape (Brassica napus L.). Z Acker Pflanzenbau 186:253–259. doi:10.1046/j.1439-037x.2001.00476.x
Moser D, Drapela T, Zaller JG, Frank T (2009) Interacting effects of wind direction and resource distribution on insect pest densities. Basic Appl Ecol 10:208–215. doi:10.1016/j.baae.2008.03.008
Müller HJ (1941) Weitere beiträge zur biologie des raspglanzkäfers, Meligethes aeneus F. (Ueber das winterlager und die massenbewegung im frühjahr). Z Pflanzenkrankh Pflanzenschutz 51:529–595
Nilsson C (1985) Impact of ploughing on emergence of pollen beetle parasitoids after hibernation. Z Angew Entomol 100:302–308
Nilsson C (1994) Pollen beetle (Meligethes aeneus) in oilseed rape crops (Brassica napus L.): biological interactions and crop losses. Dissertation, Swedish University of Agricultural Sciences
Nilsson C, Buchs W, Klukowski Z, Luik A, Ulber B, Williams I (2015) Integrated crop and pest management of winter oilseed rape (Brassica napus L.). Zemdirbyste 102:325–334
Nitzsche O, Ulber B (1998) Influence of different tillage treatments following the harvest of oilseed rape on the mortality of pollen beetles (Meligethes spp.) parasitoids. Z Pflanzenkrankh Pflanzenschutz 105:417–421
ÖBG (2001) Bodenaufnahme Systeme in Österreich. Mitt Österr Bodenkd Ges 62:219
Ouvrard P, Hicks DM, Mouland M, Nicholls JA, Baldock KC, Goddard MA, Kunin WE, Potts SG, Thieme T, Veromann E (2016) Molecular taxonomic analysis of the plant associations of adult pollen beetles (Nitidulidae: Meligethinae), and the population structure of Brassicogethes aeneus. Genome 59:1101–1116
Podlaska J, Markus J, Dmoch J, Loboda T, Pietkiewicz S, Lewandowski M (1996) Compensation of damage caused by pollen beetle (Meligethes aeneus) on winter oilseed rape under different plant density and fertilisation: I. some morphological characteristics. Rośliny Oleiste 17:325–330
Robinson RA, Sutherland WJ (2002) Post-war changes in arable farming and biodiversity in Great Britain. J Appl Ecol 39:157–176. doi:10.1046/j.1365-2664.2002.00695.x
Robinson KA, Jonsson M, Wratten SD, Wade MR, Buckley HL (2008) Implications of floral resources for predation by an omnivorous lacewing. Basic Appl Ecol 9:172–181. doi:10.1016/j.baae.2007.01.002
Rusch A, Valantin-Morison M (2013) Effect of nitrogen fertilization, cultivar and species on incidence of two major pests of winter oilseed rape (Brassica napus L.): the pollen beetle (Meligethes aeneus F.) and the stem weevil (Ceutorhynchus napi Gyl.). IOBC/WPRS Bull 92:39–44
Rusch A, Valantin-Morison M, Sarthou JP, Roger-Estrade J (2010) Integrating crop and landscape management into new crop protection strategies to enhance biological control of oilseed rape pests. In: Williams IH (ed) Biocontrol-based intergrated management of oilseed rape pests. Springer, London, pp 415–448
Rusch A, Valantin-Morison M, Roger-Estrade J, Sarthou J-P (2012) Local and landscape determinants of pollen beetle abundance in overwintering habitats. Agric Forest Entomol 14:37–47. doi:10.1111/j.1461-9563.2011.00547.x
Rusch A, Suchail S, Valantin-Morison M, Sarthou J-P, Roger-Estrade J (2013a) Nutritional state of the pollen beetle parasitoid Tersilochus heterocerus foraging in the field. Biocontrol 58:17–26. doi:10.1007/s10526-012-9463-1
Rusch A, Valantin-Morison M, Sarthou JP, Roger-Estrade J (2013b) Effect of crop management and landscape context on insect pest populations and crop damage. Agric Ecosyst Environ 166:118–125. doi:10.1016/j.agee.2011.05.004
Schlinkert H, Westphal C, Clough Y, Ludwig M, Kabouw P, Tscharntke T (2015) Feeding damage to plants increases with plant size across 21 Brassicaceae species. Oecologia 179:455–466. doi:10.1007/s00442-015-3353-z
Schlinkert H, Westphal C, Clough Y, Grass I, Helmerichs J, Tscharntke T (2016) Plant size affects mutualistic and antagonistic interactions and reproductive success across 21 Brassicaceae species. Ecosphere 7:e01529. doi:10.1002/ecs2.1529
Schmidt MH, Roschewitz I, Thies C, Tscharntke T (2005) Differential effects of landscape and management on diversity and density of ground-dwelling farmland spiders. J Appl Ecol 42:281–287. doi:10.1111/j.1365-2664.2005.01014.x
Schneider G, Krauss J, Riedinger V, Holzschuh A, Steffan-Dewenter I (2015) Biological pest control and yields depend on spatial and temporal crop cover dynamics. J Appl Ecol 52:1283–1292. doi:10.1111/1365-2664.12471
Skellern MP, Cook SM (in press) Prospects for improved habitat management for pollen beetle control. Arthropod Plant Interact
Speight MR, Lawton JH (1976) The influence of weed-cover on the mortality imposed on artificial prey by predatory ground beetles in cereal fields. Oecologia 23:211–223. doi:10.1007/bf00361237
Stoate C, Boatman ND, Borralho RJ, Rio Carvalho C, de Snoo GR, Eden P (2001) Ecological impacts of arable intensification in Europe. J Environ Manag 63:337–365
Sunderland K, Samu F (2000) Effects of agricultural diversification on the abundance, distribution, and pest control potential of spiders: a review. Entomol Exp Appl 95:1–13. doi:10.1046/j.1570-7458.2000.00635.x
Tatchell GM (1983) Compensation of spring-sown oilseed rape (Brassica napus L.) plants in response to injury to their flower buds and pods. J Agric Sci 101:565–573
Thieme T, Heimbach U, Muller A (2010) Chemical control of insect pests and insecticide resistance in oilseed rape. In: Williams IH (ed) Biocontrol-based integrated management of oilseed rape pests. Springer, London, pp 131–335
Thies C, Steffan-Dewenter I, Tscharntke T (2008) Interannual landscape changes influence plant–herbivore–parasitoid interactions. Agric Ecosyst Environ 125:266–268. doi:10.1016/j.agee.2007.12.011
Townsend TJ, Ramsden SJ, Wilson P (2016) How do we cultivate in England? Tillage practices in crop production systems. Soil Use Manag 32:106–117. doi:10.1111/sum.12241
Ulber B, Nitzsche O (2006) Phenology of parasitoids (Hym., Ichneumonidae-Tersilochinae) of oilseed rape pests in Northern Germany in 1995–1997. IOBC/WPRS Bull 29:173–179
Ulber B, Klukowski Z, Williams IH (2010a) Impact of insecticides on parasitoids of oilseed rape pests. In: Williams IH (ed) Biocontrol-based integrated management of oilseed rape pests. Springer, London, pp 337–356
Ulber B, Williams IH, Klukowski Z, Luik A, Nilsson C (2010b) Parasitoids of oilseed rape pests in Europe: key species for conservation biocontrol. In: Williams IH (ed) Biocontrol-based integrated management of oilseed rape pests. Springer, London, pp 45–76
Valantin-Morison M, Meynard J-M, Doré T (2007) Effects of crop management and surrounding field environment on insect incidence in organic winter oilseed rape (Brassica napus L.). Crop Prot 26:1108–1120
Vanbergen AJ (2013) Threats to an ecosystem service: pressures on pollinators. Front Ecol Environ 11:251–259. doi:10.1890/120126
Vasak J (1983) On the dynamics of the form and reduction of yield components in winter rape (Brassica napus L.). In: Proccedings of the 6th International Rapeseed Conference, Paris, France, pp 303–303
Vasileiadis VP, Sattin M, Otto S, Veres A, Pálinkás Z, Ban R, Pons X, Kudsk P, van der Weide R, Czembor E, Moonen AC, Kiss J (2011) Crop protection in European maize-based cropping systems: current practices and recommendations for innovative integrated pest management. Agric Syst 104:533–540. doi:10.1016/j.agsy.2011.04.002
Veromann E, Metspalu L, Williams IH, Hiiesaar K, Mand M, Kaasik R, Kovacs G, Jogar K, Svilponis E, Kivimagi I, Ploomi A, Luik A (2012) Relative attractiveness of Brassica napus, Brassica nigra, Eruca sativa and Raphanus sativus for pollen beetle (Meligethes aeneus) and their potential for use in trap cropping. Arthropod Plant Interact 6:385–394. doi:10.1007/s11829-012-9191-6
Veromann E, Toome M, Kanaste A, Kaasik R, Copolovici L, Flink J, Kovacs G, Narits L, Luik A, Niinemets U (2013) Effects of nitrogen fertilization on insect pests, their parasitoids, plant diseases and volatile organic compounds in Brassica napus. Crop Prot 43:79–88. doi:10.1016/j.cropro.2012.09.001
Vinatier F, Gosme M, Valantin-Morison M (2012) A tool for testing integrated pest management strategies on a tritrophic system involving pollen beetle, its parasitoid and oilseed rape at the landscape scale. Landsc Ecol 27:1421–1433. doi:10.1007/s10980-012-9795-3
Vinatier F, Gosme M, Valantin-Morison M (2013) Explaining host–parasitoid interactions at the landscape scale: a new approach for calibration and sensitivity analysis of complex spatio-temporal models. Landsc Ecol 28:217–231. doi:10.1007/s10980-012-9822-4
Wahmhoff W, Hedke K, von Tiedemann A, Nitzsche O, Ulber B (1999) Impact of crop rotation and soil cultivation on the development of pests and diseases of rapeseed. Z Pflanzenkrankh Pflanzenschutz 106:57–73
Williams IH (2004) Advances in insect pest management of oilseed rape in Europe. In: Ishaaya I, Horowitz AR (eds) Novel approaches to insect pest management. Springer, Berlin, pp 181–208
Williams IH (2006) Integrating parasitoids into management of pollen beetle on oilseed rape. Agron Res 4:465–470
Williams IH (2010) The major insect pests of oilseed rape in Europe and their management: an overview. In: Williams IH (ed) Biocontrol-based integrated management of oilseed rape pests. Springer, London, pp 1–43
Williams IH, Ferguson AW (2008) Oilseed rape crop ecology: optimising crop husbandry for conservation biological control and greater biodiversity: Final Project report for Defra Project AR0316. http://sciencesearch.defra.gov.uk/Default.aspx?Menu=Menu&Module=More&Location=None&Completed=0&ProjectID=13058#Description. Accessed January 2017
Williams IH, Free JB (1979) Compensation of oilseed rape (Brassica napus L.) plants after damage to their buds and pods. J Agic Sci 92:53–59
Williams IH, Ferguson AW, Kruus M, Veromann E, Warner DJ (2010) Ground beetles as predators of oilseed rape pests: incidence, spatio-temporal distributions and feeding. In: Williams IH (ed) Biocontrol-based integrated management of oilseed rape pests. Springer, London, pp 115–150
Winkler K, Wäckers FL, Bukovinszkine-Kiss G, van Lenteren J (2006) Sugar resources are vital for Diadegma semiclausum fecundity under field conditions. Basic Appl Ecol 7:133–140. doi:10.1016/j.baae.2005.06.001
Zaller JG, Moser D, Drapela T, Schmöger C, Frank T (2008a) Effect of within-field and landscape factors on insect damage in winter oilseed rape. Agric Ecosyst Environ 123:233–238. doi:10.1016/j.agee.2007.07.002
Zaller JG, Moser D, Drapela T, Schmöger C, Frank T (2008b) Insect pests in winter oilseed rape affected by field and landscape characteristics. Basic Appl Ecol 9:682–690. doi:10.1016/j.baae.2007.10.004
Zaller J, Moser D, Drapela T, Schmoger C, Frank T (2009a) Parasitism of stem weevils and pollen beetles in winter oilseed rape is differentially affected by crop management and landscape characteristics. Biocontrol 54:505–514
Zaller JG, Moser D, Drapela T, Schmoeger C, Frank T (2009b) Parasitism of stem weevils and pollen beetles in winter oilseed rape is differentially affected by crop management and landscape characteristics. Biocontrol 54:505–514. doi:10.1007/s10526-009-9212-2
Zimmer CT, Köhler H, Nauen R (2014) Baseline susceptibility and insecticide resistance monitoring in European populations of Meligethes aeneus and Ceutorhynchus assimilis collected in winter oilseed rape. Entomol Exp Appl 150:279–288. doi:10.1111/eea.12162
Zlof V (2008) Recommendations and conclusions of the Ad hoc EPPO Workshop on insecticide resistance of Meligethes spp. (pollen beetle) on oilseed rape. EPPO Bulletin 38:65–67. doi:10.1111/j.1365-2338.2008.01182.x
Acknowledgements
This review was funded by the UK Defra Health & Safety Executive (Chemicals Regulation Directorate) Project PS2141. Rothamsted Research receives strategic funding from the UK Biotechnology and Biological Sciences Research Council (BBSRC). SMC is part-supported by research programme NE/N018125/1 LTS-M ASSIST - Achieving Sustainable Agricultural Systems, funded by UK BBSRC and NERC and UK BBSRC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Heikki Hokkanen.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Skellern, M.P., Cook, S.M. The potential of crop management practices to reduce pollen beetle damage in oilseed rape. Arthropod-Plant Interactions 12, 867–879 (2018). https://doi.org/10.1007/s11829-017-9571-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-017-9571-z