Abstract
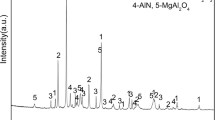
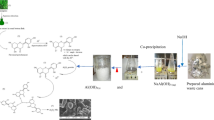
Nano γ-alumina was produced using Nepheline syenite ore by leaching and precipitation process at a certain pH in the presence of sodium dodecyl sulfate (SDS) as a surfactant. The produced nanostructure was characterized by XRD, SEM, EDX, DLS, BET and FT-IR. The XRD pattern confirmed the tetragonal structure of alumina The nano structure of alumina was approved by SEM and the particle size distribution were between 41 to 486 nm, confirmed by DLS. BET analysis showed that the specific surface area of nanopowder was about 39.1 m2/g. The synthesis conditions were modeled and optimized by RSM. The optimum conditions resulted in leaching time, the mass ratio of Nepheline/HCl, and the reflux temperature of 2 h, 20, and 70 °C, respectively. Under optimum conditions, the extraction efficiency was 82%. The prepared nano γ-alumina has higher removal efficiency than commercial types in the removal of p-nitrophenol by adsorption process.
Similar content being viewed by others
References
K. Davis, Material Review: Alumina (Al2O3), School of Doctoral Studies European Union Journal (2010).
F. Pan, X. Lu, T. Wang, Y. Wang, Z. Zhang, Y. Yan and S. Yang, Mater. Lett., 91, 136 (2013).
W. Li, Q. Jia, Z. Zhang and Y. Wang, Korean J. Chem. Eng., 33, 1337 (2016).
M.W. Tamele, Discussions of the Faraday Society, 8, 270 (1950).
J. E. Park, B.B. Kim and E.D. Park, Korean J. Chem. Eng., 32, 2212 (2015).
S. Wang, X. Li, S. Wang, Y. Li and Y. Zhai, Mater. Lett., 62, 3552 (2008).
G. Paglia, A. L. Rohl, C. E. Buckley and J.D. Gale, Phys. Rev. B, 71, 224115 (2005).
B. L. Cushing, V. L. Kolesnichenko and C. J. O’Connor, Chem. Rev., 104, 3893 (2004).
M. Niederberger and N. Pinna, Metal oxide nanoparticles in organic solvents: synthesis, formation, assembly and application, Springer Science & Business Media (2009).
K. Byrappa and M. Yoshimura, Handbook of hydrothermal technology, William Andrew (2012).
Y. Kim, B. Lee and J. Yi, Korean J. Chem. Eng., 19, 908 (2002).
S.A. Hosseini, Int. J. Mater. Chem. Phys., 1(2), 93 (2015).
F. Pan, X. Lu, T. Wang, Y. Wang, Z. Zhang and Y. Yan, Appl. Clay Sci., 85, 31 (2013).
L. Qu, C. He, Y. Yang, Y. He and Z. Liu, Mater. Lett., 59, 4034 (2005).
M. Ezoddin, F. Shemirani, K. Abdi, M.K. Saghezchi and M. Jamali, J. Hazard. Mater., 178, 900 (2010).
K. Parida, A. C. Pradhan, J. Das and N. Sahu, Mater. Chem. Phys., 113, 244 (2009).
N. Majidian, N. Habibi and M. Rezaei, Korean J. Chem. Eng., 31, 1162 (2014).
H. Yang, M. Liu and J. Ouyang, Appl. Clay Sci., 47, 438 (2010).
A.R. Ferreira, E. Küçükbenli, S. De Gironcoli, W. F. Souza, S. S. X. Chiaro, E. Konstantinova and A. A. Leitão, Chem. Phys., 423, 62 (2013).
T. H. Ballinger and J.T. Yates Jr., Langmuir, 7, 3041 (1991).
L. Sicard, P. L. Llewellyn, J. Patarin and F. Kolenda, Micropor. Mesopor. Mater., 44-45, 195 (2001).
J.C. Ray, K.-S. You, J.-W. Ahn and W.-S. Ahn, Micropor. Mesopor. Mater., 100, 183 (2007).
M. Zabeti, W. M. A.W. Daud and M. K. Aroua, Appl. Catal. A: Gen., 366, 154 (2009).
M.N. Chong, H. Zhu and B. Jin, Chem. Eng. J., 156, 278 (2010).
X. Han, Y. He, H. Zhao and D. Wang, Korean J. Chem. Eng., 31, 1810 (2014).
W. Wang, H. Ma, W. Xu, L. Gong, W. Zhang and D. Zou, Biochem. Eng. J., 39, 604 (2008).
A.R. Khataee, M. Zarei and L. Moradkhannejhad, Desalination, 258, 112 (2010).
L. Vordonis, P. G. Koutsoukos and A. Lycourghiotis, Colloids Surf., 50, 353 (1990).
J. Xiao, L. Zhao, W. Zhang, X. Liu and Y. Chen, Korean J. Chem. Eng., 31, 253 (2014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chitan, M., Hosseini, S.A., Salari, D. et al. Synthesis of γ-alumina nano powder from Nepheline syenite. Korean J. Chem. Eng. 34, 66–72 (2017). https://doi.org/10.1007/s11814-016-0246-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-016-0246-8